Evaluation of Antioxidant Activity in Traditional Indonesian Herbal Medicine (Jamu) Using ABTS and DPPH Testing Methods
DOI:
https://doi.org/10.12928/jafost.v6i4.13005Keywords:
ABTS, Antioxidant activity, DPPH, Herbal medicine, JamuAbstract
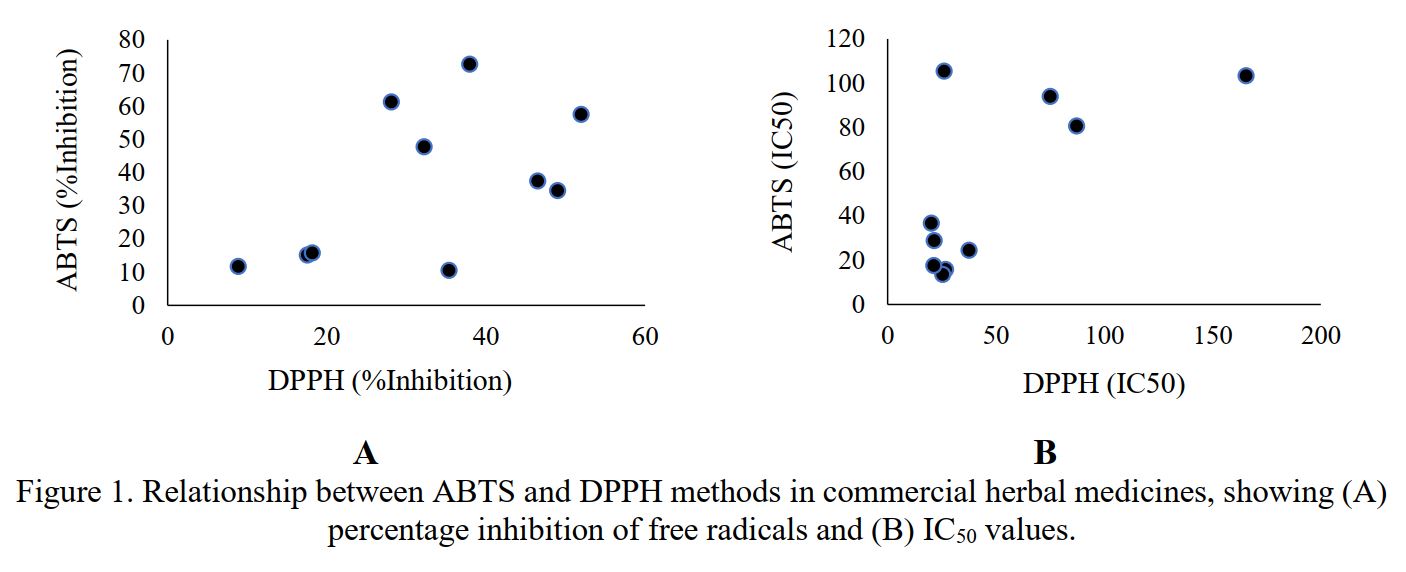
Herbal medicines are widely consumed as natural antioxidants to prevent oxidative stress-related diseases. Accurate assessment of antioxidant activity is essential, yet different methods may yield varying results. This study contributed to compare the antioxidant activities of ten commercial herbal medicines using ABTS (2,2’-azinobis- (3-ethylbenzothiazoline-6-sulfonic acid) and DPPH (2,2-diphenyl-1picrylhydrazyl) assays and evaluate the correlation between both methods. Ten herbal formulations were collected from local producers in Samarinda, Indonesia. Each product was mixed with low-fat milk (1:10 w/v) to enhance bio-accessibility and extracted via sonication and centrifugation. Antioxidant activity was analyzed using ABTS and DPPH radical scavenging assays. Percentage inhibition and IC₅₀ values were calculated. Statistical analysis was performed using GraphPad Prism 9.5.0 with Pearson correlation at a 95% confidence level. ABTS inhibition ranged from 10.54% to 72.73%, while DPPH ranged from 8.89% to 49.03%. IC₅₀ values were lower in ABTS (13.51–105.36 µg/mL) than in DPPH (20.11–165.50 µg/mL). A moderate positive correlation (r=0.5390) between inhibition results was observed but was not statistically significant (p=0.1079). Among all samples, the herbal formulation containing turmeric, betel leaf, areca nut, and manjakani exhibited the highest antioxidant activity with 72.73% inhibition (ABTS) and an IC₅₀ of 13.51 µg/mL. The differing sensitivities of ABTS and DPPH assays suggest that both methods should be used complementarily to obtain a comprehensive antioxidant profile of herbal products.
References
E. Niki, “Lipid oxidation that is, and is not, inhibited by vitamin E: Consideration about physiological functions of vitamin E,” Free Radic. Biol. Med., vol. 176, pp. 1–15, 2021, https://doi.org/10.1016/j.freeradbiomed.2021.09.001.
L. Valgimigli, “Lipid peroxidation and antioxidant protection,” Biomolecules, vol. 13, no. 9, p. 1291, 2023, https://doi.org/10.3390/biom13091291.
Y. Saito, “Lipid peroxidation products as a mediator of toxicity and adaptive response – The regulatory role of selenoprotein and vitamin E,” Arch. Biochem. Biophys., vol. 703, p. 108840, 2021, https://doi.org/10.1016/j.abb.2021.108840.
E. Usoro, “Comparative analysis of natural vs. synthetic antioxidants in Nigeria,” J. Chem., vol. 3, no. 3, pp. 27–37, 2024, https://doi.org/10.47672/jchem.2515.
P. Chaudhary, P. Janmeda, A. O. Docea, B. Yeskaliyeva, A. F. Abdull Razis, B. Modu, D. Calina, and J. Sharifi-Rad, “Oxidative stress, free radicals and antioxidants: Potential crosstalk in the pathophysiology of human diseases,” Front. Chem., vol. 11, 2023, https://doi.org/10.3389/fchem.2023.1158198.
M. Viana da Silva, M. R. C. Santos, I. R. Alves Silva, E. B. Macedo Viana, D. A. Dos Anjos, I. A. Santos, N. G. Barbosa de Lima, C. Wobeto, N. Jorge, and S. C. D. S. Lannes, “Synthetic and natural antioxidants used in the oxidative stability of edible oils: An overview,” Food Rev. Int., vol. 38, no. sup1, pp. 349–372, 2022, https://doi.org/10.1080/87559129.2020.1869775.
I. G. Munteanu and C. Apetrei, “Analytical methods used in determining antioxidant activity: A review,” Int. J. Mol. Sci., vol. 22, no. 7, p. 3380, 2021, https://doi.org/10.3390/ijms22073380.
F. Husain, E. Yuniati, A. A. Arsi, H. Wicaksono, and B. F. Wahidah, “Ethnobotanical knowledge on jamu herbal drink among consumer in Semarang,” IOP Conf. Ser. Earth Environ. Sci., vol. 743, no. 1, p. 012019, 2021, https://doi.org/10.1088/1755-1315/743/1/012019.
T. Estiasih, J. M. Maligan, J. E. Witoyo, A. A. H. Mu’alim, K. Ahmadi, T. Mahatmanto, and E. Zubaidah, “Indonesian traditional herbal drinks: Diversity, processing, and health benefits,” J. Ethn. Foods, vol. 12, no. 1, p. 7, 2025, https://doi.org/10.1186/s42779-025-00267-5.
N. P. E. Trisdayanti and I. M. P. D. Atmaja, “Moringa jamu (recipe formulation, hedonic test and nutrition),” J. Gastron. Tour., vol. 9, no. 1, pp. 27–33, 2022, https://doi.org/10.17509/gastur.v9i1.48278.
T. Kartika, B. Suharti, and S. Sugiyanta, “Jamu as herbal medicine: A study of health communication and philosophy as cultural identity,” KOMUNIKA, vol. 6, no. 1, 2023, https://doi.org/10.24042/komunika.v6i1.16247.
W. Nurcholis and R. Arianti, “Jamu as Indonesian cultural heritage and modern health innovation,” J. Jamu Indones., vol. 9, no. 1, pp. 1–2, 2024, https://doi.org/10.29244/jji.v9i1.317.
W. D. Fitriana, S. B. T. Istiqomah, T. Ersam, and S. Fatmawati, “The relationship of secondary metabolites: A study of Indonesian traditional herbal medicine (Jamu) for post partum maternal care use,” in The 3rd International Seminar on Chemistry: Green Chemistry and Its Role for Sustainability, 2018, p. 020096. https://doi.org/10.1063/1.5082501.
S. Ortiz-Islas, C. A. Espinosa-Leal, T. González-Rodríguez, and S. García-Lara, “Enhancing the antioxidant activity of tea (Camellia sinensis) through common herbal infusions,” Foods, vol. 13, no. 20, p. 3284, 2024, https://doi.org/10.3390/foods13203284.
X. Li, L. Wu, R. Wu, M. Sun, K. Fu, T. Kuang, and Z. Wang, “Comparison of medicinal preparations of Ayurveda in India and five traditional medicines in China,” J. Ethnopharmacol., vol. 284, p. 114775, 2022, https://doi.org/10.1016/j.jep.2021.114775.
X. Zhang, H. Qiu, C. Li, P. Cai, and F. Qi, “The positive role of traditional Chinese medicine as an adjunctive therapy for cancer,” Biosci. Trends, vol. 15, no. 5, p. 2021.01318, 2021, https://doi.org/10.5582/bst.2021.01318.
X. Li, M. Han, X. Song, M. Sun, L. Xu, Y. Liang, Y. Shen, Y. Song, J. Zhang, W. Chen, M. Zhao, L. Wu, D. Hu, M. He, T. Tian, et al., “Characteristics and comparative study of medicinal materials between China and India based on data mining from literatures,” J. Ethnopharmacol., vol. 333, p. 118409, 2024, https://doi.org/10.1016/j.jep.2024.118409.
F. Yunita, S. Gunawan, H. Silaban, and Chaidir, “The journey of Indonesian traditional medicine,” Tarumanagara Med. J., vol. 6, no. 2, pp. 241–252, 2024, https://doi.org/10.24912/tmj.v6i2.33351.
M. Platzer, S. Kiese, T. Herfellner, U. Schweiggert-Weisz, O. Miesbauer, and P. Eisner, “Common trends and differences in antioxidant activity analysis of phenolic substances using single electron transfer based assays,” Molecules, vol. 26, no. 5, p. 1244, 2021, https://doi.org/10.3390/molecules26051244.
J. Rumpf, R. Burger, and M. Schulze, “Statistical evaluation of DPPH, ABTS, FRAP, and Folin-Ciocalteu assays to assess the antioxidant capacity of lignins,” Int. J. Biol. Macromol., vol. 233, p. 123470, 2023, https://doi.org/10.1016/j.ijbiomac.2023.123470.
G. Ren, Y. Sun, M. Li, J. Ning, and Z. Zhang, “Cognitive spectroscopy for evaluating Chinese black tea grades (Camellia sinensis): Near‐infrared spectroscopy and evolutionary algorithms,” J. Sci. Food Agric., vol. 100, no. 10, pp. 3950–3959, 2020, https://doi.org/10.1002/jsfa.10439.
A. A. Akande, U. Salar, K. M. Khan, S. Syed, S. A. Aboaba, S. Chigurupati, A. Wadood, M. Riaz, M. Taha, S. Bhatia, Kanwal, S. Shamim, and S. Perveen, “Substituted benzimidazole analogues as potential α-amylase inhibitors and radical scavengers,” ACS Omega, vol. 6, no. 35, pp. 22726–22739, 2021, https://doi.org/10.1021/acsomega.1c03056.
M. O. Allison, V. C. Ukor, Y. A. Ibrahim, and J. A. Adeyemo, “Leveraging natural antioxidants in disease prevention: Investigating the role of medicinal herbs in reducing oxidative stress and chronic disease,” World J. Adv. Res. Rev., vol. 25, no. 2, pp. 2720–2733, 2025, https://doi.org/10.30574/wjarr.2025.25.2.0593.
R. Wołosiak, B. Drużyńska, D. Derewiaka, M. Piecyk, E. Majewska, M. Ciecierska, E. Worobiej, and P. Pakosz, “Verification of the conditions for determination of antioxidant activity by ABTS and DPPH assays—A practical approach,” Molecules, vol. 27, no. 1, p. 50, 2021, https://doi.org/10.3390/molecules27010050.
I. R. A. Sasmita, M. A. Suryanegara, M. W. Apriliyanti, D. M. Yusuf, and F. W. R. Ana, “Effects of time and temperature variations on curcumin contents and antioxidant activity of tamarind-turmeric herbs,” IOP Conf. Ser. Earth Environ. Sci., vol. 672, no. 1, p. 012052, 2021, https://doi.org/10.1088/1755-1315/672/1/012052.
N. W. Sudatri, G. A. M. K. Dewi, I. G. Mahardika, and I. G. N. G. Bidura, “Kidney histology and broiler serum creatinine levels supplemented with a mixture of water extract of turmeric and tamarind fruit,” Int. J. Fauna Biol. Stud., vol. 8, no. 1, pp. 95–100, 2021, https://doi.org/10.22271/23940522.2021.v8.i1b.799.
X. Zeng, H. Tan, B. Liu, and Y. Wen, “Optimization of ultrasonic-assisted extraction and purification of total flavonoids with biological activities from Radix Puerariae,” Biomass Convers. Biorefinery, vol. 14, no. 24, pp. 31533–31545, 2024, https://doi.org/10.1007/s13399-023-04921-3.
L. Niang, S. A. Mahamat, N. C. Ayessou, M. Cisse, and C. M. Diop, “Antioxidant activity of hydro-acetonic, hydro-methanolic and aqueous leaf and bark extracts of Sclerocaria birrea (A. Rich.) hochst,” Food Nutr. Sci., vol. 12, no. 05, pp. 429–438, 2021, https://doi.org/10.4236/fns.2021.125033.
K. Abdelouhab, T. Guemmaz, M. Karamać, D. E. Kati, R. Amarowicz, and L. Arrar, “Phenolic composition and correlation with antioxidant properties of various organic fractions from Hertia cheirifolia extracts,” J. Pharm. Biomed. Anal., vol. 235, p. 115673, 2023, https://doi.org/10.1016/j.jpba.2023.115673.
M. Mitterer-Ddaltoé, J. Bordim, C. Lise, L. Breda, M. Casagrande, and V. Lima, “Consumer awareness of food antioxidants. Synthetic vs. Natural,” Food Sci. Technol., vol. 41, no. suppl 1, pp. 208–212, 2021, https://doi.org/10.1590/fst.15120.
S. Abbas, S. M. Latif, I. I. Muhamad, M. A. Hesan, and F. Kormin, “In vitro antioxidant and anticholinesterase activities of ethanolic turmeric crude extract,” Food Res., vol. 6, no. 4, pp. 199–204, 2022, https://doi.org/10.26656/fr.2017.6(4).101.
J. Sukweenadhi, O. Yunita, F. Setiawan, K, Kartini, M. T. Siagian, N. P. Danduru, and C. Avanti, “Antioxidant activity screening of seven Indonesian herbal extract,” Biodiversitas J. Biol. Divers., vol. 21, no. 5, 2020, https://doi.org/10.13057/biodiv/d210532.
M. Parcheta, R. Świsłocka, S. Orzechowska, M. Akimowicz, R. Choińska, and W. Lewandowski, “Recent developments in effective antioxidants: The structure and antioxidant properties,” Materials (Basel)., vol. 14, no. 8, p. 1984, 2021, https://doi.org/10.3390/ma14081984.
T. R. Kıran, O. Otlu, and A. B. Karabulut, “Oxidative stress and antioxidants in health and disease,” J. Lab. Med., vol. 47, no. 1, pp. 1–11, 2023, https://doi.org/10.1515/labmed-2022-0108.
G. Slaček, P. Kotnik, A. Osmić, V. Postružnik, Ž. Knez, M. Finšgar, and M. Knez Marevci, “The extraction process, separation, and identification of curcuminoids from turmeric curcuma longa,” Foods, vol. 12, no. 21, p. 4000, 2023, https://doi.org/10.3390/foods12214000.
C. Wu, H. Dong, P. Wang, M. Han, and X. Xu, “Sequential changes in antioxidant activity and structure of curcumin-myofibrillar protein nanocomplex during in vitro digestion,” Food Chem., vol. 382, p. 132331, 2022, https://doi.org/10.1016/j.foodchem.2022.132331.
A. Mouithys-Mickalad, K. S. Etsè, T. Franck, J. Ceusters, A. Niesten, H. Graide, G. Deby-Dupont, C. Sandersen, and D. Serteyn, “Free radical inhibition using a water-soluble curcumin complex, NDS27: Mechanism study using EPR, chemiluminescence, and docking,” Antioxidants, vol. 13, no. 1, p. 80, 2024, https://doi.org/10.3390/antiox13010080.
N. Darsini, “Short communication: The species of Temu-temuan that sold in Badung Market with its utilization and anatomical study,” J. Biol. Udayana, vol. 26, no. 2, p. 285, 2022, https://doi.org/10.24843/JBIOUNUD.2022.v26.i02.p14.
I. Widyastuti, H. Z. Luthfah, Y. I. Hartono, R. Islamadina, A. T. Can, and A. Rohman, “Antioxidant activity of temulawak (Curcuma xanthorrhiza Roxb.) and its classification with chemometrics,” Indones. J. Chemom. Pharm. Anal., p. 29, 2020, https://doi.org/10.22146/ijcpa.507.
I. R. Ilyasov, V. L. Beloborodov, I. A. Selivanova, and R. P. Terekhov, “ABTS/PP decolorization assay of antioxidant capacity reaction pathways,” Int. J. Mol. Sci., vol. 21, no. 3, p. 1131, 2020, https://doi.org/10.3390/ijms21031131.
N. Chaves, A. Santiago, and J. C. Alías, “Quantification of the antioxidant activity of plant extracts: Analysis of sensitivity and hierarchization based on the method used,” Antioxidants, vol. 9, no. 1, p. 76, 2020, https://doi.org/10.3390/antiox9010076.
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Rimbawan Apriyadi, Kartika Sari, Maulida Rachmawati, Muhammad Rafii Nur Fauzan, Miftakhur Rohmah

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Authors who publish with the Journal of Agri-food Science and Technology (JAFOST) agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License (CC BY-SA 4.0) that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial publication in this journal.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work.



