Exploration of Instant Functional Drinks Based on Angkak and Kidney Bean Flour: Implications on Bioactive Compound and Antioxidant Activity
DOI:
https://doi.org/10.12928/jafost.v6i3.12856Keywords:
Antioxidant, Angkak, Functional drinks, Instant powder drink, Kidney bean flourAbstract
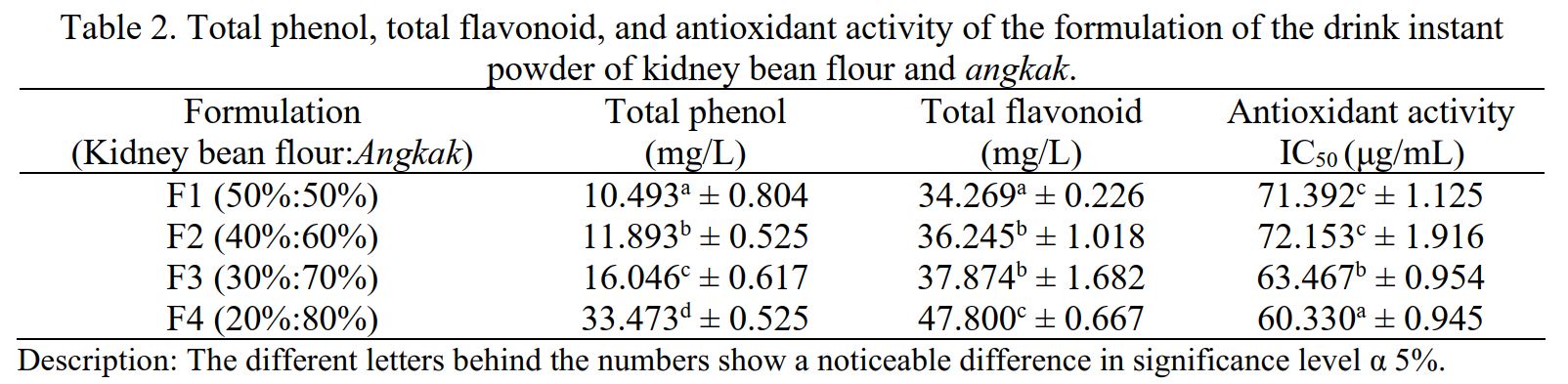
Indonesian eating patterns have shifted to more fast food, which is high in fat and free radicals. Free radicals are unstable and highly reactive molecules and will react with surrounding molecules to obtain electron pairs. Angkak and kidney bean flour contain bioactive compounds such as alkaloids, phenols, terpenoids, triterpenoids, flavonoids, isoflavonoids, flobatananis, coumarins, and saponins that could act as antioxidants. Both food ingredients can be modified into functional instant drinks to increase their benefits in providing daily antioxidant intake. Instant powder drinks are one of the alternative ways to get the benefits of plants or food. They have a pharmacological effect health, as they are used or consumed more easily and practically. This study aimed to contribute to producing an instant functional drink high in bioactive compounds and antioxidants as an alternative functional food. The research design used was a single-factor Complete Randomized Design (CRD) with four treatments, each repeated 3 times, so that 12 experimental units were obtained. The treatment in the study of making instant powder drinks consisting of the ratio of angkak and kidney bean flour was P1 (50:50%), P2 (40:60%), P3 (30:70%), and P4 (20:80%). Phenol, flavonoid, and antioxidant activity values were highest successively in the F4 treatment of 31.895 mg/L, 47.800 mg/L, and 60.996 μg/ml. The results show that instant powder drinks of kidney bean flour and angkak are included in the strong category and can be used as functional drinks high in antioxidants.
References
N. Andarwulan, S. Madanijah, D. Briawan, K. Anwar, A. Bararah, Saraswati, and D. Średnicka-Tober, “Food consumption pattern and the intake of sugar, salt, and fat in the South Jakarta City—Indonesia,” Nutrients, vol. 13, no. 4, pp. 1–19, 2021, https://doi.org/10.3390/nu13041289.
A. Panou and I. K. Karabagias, “Composition, properties, and beneficial effects of functional beverages on human health,” Beverages, vol. 11, no. 2, pp. 3–5, 2025, https://doi.org/10.3390/beverages11020040.
S. Katz and S. Katz, “Cardiovascular disease - Is a whole food plant-based diet the answer ?,” The Science Journal of the Lander College of Arts and Sciences, vol. 15, no. 2, pp. 61–69, 2022. https://touroscholar.touro.edu/sjlcas/vol15/iss2/11.
BSN, “SNI 01-4320-1996 Serbuk Minuman Tradisional,” 1996, Badan Standarisasi Nasional.
E. Palupi, N. Delina, N. M. Nurdin, H. F. Navratilova, R. Rimbawan, and A. Sulaeman, “Kidney bean substitution ameliorates the nutritional quality of extruded purple sweet potatoes: Evaluation of chemical composition, glycemic index, and antioxidant capacity,” Foods, vol. 12, no. 7, 2023, https://doi.org/10.3390/foods12071525.
M. I. Syafutri, F. Syaiful, E. Lidiasari, P. Parwiyanti, S. Sugito, E. I. Astari, and J. M. Saputra, “Characteristics of composite flour made of kidney bean and soybean,” J. Appl. Agric. Sci. Technol., vol. 7, no. 2, pp. 119–129, 2023, https://doi.org/10.55043/jaast.v7i2.132.
N. Ramadani Fitri and A. Puspitarini Siswanto, “Formulation of instant powder drink combination of red ginger and banana peel,” Mater. Today Proc., vol. 87, pp. 101–105, 2023, https://doi.org/10.1016/j.matpr.2023.02.379.
S. Martínez, C. Fuentes, and J. Carballo, “Antioxidant Activity, total phenolic content and total flavonoid content in sweet chestnut (Castanea sativa Mill.) cultivars grown in Northwest Spain under different environmental conditions,” Foods, vol. 11, no. 21, 2022, https://doi.org/10.3390/foods11213519.
M. Kumari, K. Akhilender Naidu, S. Vishwanatha, K. Narasimhamurthy, and G. Vijayalakshmi, “Safety evaluation of Monascus purpureus red mould rice in albino rats,” Food Chem. Toxicol., vol. 47, no. 8, pp. 1739–1746, 2009, https://doi.org/10.1016/j.fct.2009.04.038.
S. A. Siddiqui, S. Khan, M. Mehdizadeh, N. A. Bahmid, D. N. Adli, T. R. Walker, R. Perestrelo, and J. S. Câmara, “Phytochemicals and bioactive constituents in food packaging - A systematic review,” Heliyon, vol. 9, no. 11, 2023, https://doi.org/10.1016/j.heliyon.2023.e21196.
S. D. Rodilla, M. Martínez-Pineda, C. Yagüe-Ruiz, and A. Vercet, “Evaluation of phenolic compounds, antioxidant activity and pigment content in emerging and traditional plant-based oils in Mediterranean gastronomy,” Int. J. Gastron. Food Sci., vol. 33, p. 100771, 2023, https://doi.org/10.1016/j.ijgfs.2023.100771.
A. E. Ebrahim, N. K. Abd El-Aziz, E. Y. T. Elariny, A. Shindia, A. Osman, W. N. Hozzein, D. H. M. Alkhalifah, and D. El-Hossary, “Antibacterial activity of bioactive compounds extracted from red kidney bean (Phaseolus vulgaris L.) seeds against multidrug-resistant Enterobacterales,” Front. Microbiol., vol. 13, no. 11, pp. 1–17, 2022, https://doi.org/10.3389/fmicb.2022.1035586.
Y. Wang, H. Gao, J. Xie, X. Li, and Z. Huang, “Effects of some flavonoids on the mycotoxin citrinin reduction by Monascus aurantiacus Li AS3.4384 during liquid-state fermentation,” AMB Express, vol. 10, no. 1, 2020, https://doi.org/10.1186/s13568-020-0962-7.
T. Singh, V. K. Pandey, K. K. Dash, S. Zanwar, and R. Singh, “Natural bio-colorant and pigments: Sources and applications in food processing,” J. Agric. Food Res., vol. 12, p. 100628, 2023, https://doi.org/10.1016/j.jafr.2023.100628.
H. Speisky, F. Shahidi, A. C. de Camargo, and J. Fuentes, “Revisiting the oxidation of flavonoids: Loss, conservation or enhancement of their antioxidant properties,” Antioxidants, vol. 11, no. 1, pp. 1–28, 2022, https://doi.org/10.3390/antiox11010133.
E. J. Llorent-Martínez, A. I. Gordo-Moreno, M. L. Fernández-de Córdova, and A. Ruiz-Medina, “Preliminary phytochemical screening and antioxidant activity of commercial Moringa oleifera food supplements,” Antioxidants, vol. 12, no. 1, 2023, https://doi.org/10.3390/antiox12010110.
A. Roy, A. Khan, I. Ahmad, S. Alghamdi, B. S. Rajab, A. O. Babalghith, M. Y. Alshahrani, S. Islam, and M. R. Islam, “Flavonoids a bioactive compound from medicinal plants and its therapeutic applications,” Biomed Res. Int., vol. 22, 2022, https://doi.org/10.1155/2022/5445291.
M. Ali Al-Mamary and Z. Moussa, “Antioxidant activity: The presence and impact of hydroxyl groups in small molecules of natural and synthetic origin,” Antioxidants - Benefits, Sources, Mech. Action, 2021, https://doi.org/10.5772/intechopen.95616.
V. K. Gupta, R. Kumria, M. Garg, and M. Gupta, “Recent updates on free radicals scavenging flavonoids: An overview,” Asian Journal of Plant Sciences, vol. 9, no. 3, pp. 108–117, 2010, https://doi.org/10.3923/ajps.2010.108.117.
H. P. Mohankumari, K. A. Naidu, K. Narasimhamurthy, and G. Vijayalakshmi, “Bioactive pigments of Monascus purpureus attributed to antioxidant, HMG-CoA reductase inhibition and anti-atherogenic functions,” Front. Sustain. Food Syst., vol. 5, pp. 1–11, 2021, https://doi.org/10.3389/fsufs.2021.590427.
V. Chaudhary, P. Katyal, J. Kaur, S. Bhatia, S. Singh, A. K. Poonia, A. K. Puniya, A. Raposo, S. Yoo, H. Han, H. A. Alturki, and A. Kumar, “Bioactive activity and safety analysis of Monascus red biopigment,” Food Biosci., vol. 57, 2024, https://doi.org/10.1016/j.fbio.2023.103523.
M. Husakova, M. Plechata, B. Branska, and P. Patakova, “Effect of a Monascus sp. red yeast rice extract on germination of bacterial spores,” Front. Microbiol., vol. 12, no. 432, pp. 1–10, 2021, https://doi.org/10.3389/fmicb.2021.686100.
W. Kongbuntad and Saenphet, “Effects of red mold rice produced from Monascus purpureus CMU002U on growth performances and antioxidant activity of Japanese quail.,” vol. 15, pp. 8–14, 2016, https://doi.org/10.3923/ijps.2016.8.14.
R. Indiarto, J. A. Herwanto, F. Filianty, E. Lembong, E. Subroto, and D. R. A. Muhammad, “Total phenolic and flavonoid content, antioxidant activity and characteristics of a chocolate beverage incorporated with encapsulated clove bud extract,” CYTA - J. Food, vol. 22, no. 1, pp. 1–15, 2024, https://doi.org/10.1080/19476337.2024.2329144.
P. Chaudhary, P. Janmeda, A. O. Docea, B. Yeskaliyeva, A. F. Abdull Razis, B. Modu, D. Calina, and J. Sharifi-Rad, “Oxidative stress, free radicals and antioxidants: potential crosstalk in the pathophysiology of human diseases,” Front. Chem., vol. 11, pp. 1–24, 2023, https://doi.org/10.3389/fchem.2023.1158198.
S. G. Tumilaar, A. Hardianto, H. Dohi, and D. Kurnia, “A comprehensive review of free radicals, oxidative stress, and antioxidants: Overview, clinical applications, global perspectives, future directions, and mechanisms of antioxidant activity of flavonoid compounds,” J. Chem., vol. 24, 2023, https://doi.org/10.1155/2024/5594386.
B. Flores-Chávez, S. Hernández-León, A. L. Guzmán-Elizalde, J. Espitia-López, and P. M. Garza-López, “Methods for determination of antioxidant capacity of traditional and emergent crops of interest in Mexico: An overview,” Sci. Agropecu., vol. 15, no. 4, pp. 593–615, 2024, https://doi.org/10.17268/sci.agropecu.2024.044.
M. C. Christodoulou, J. C. Orellana Palacios, G. Hesami, S. Jafarzadeh, J. M. Lorenzo, R. Domínguez, A. Moreno, and M. Hadidi, “Spectrophotometric methods for measurement of antioxidant activity in food and pharmaceuticals,” Antioxidants, vol. 11, no. 11, 2022, https://doi.org/10.3390/antiox11112213.
S. Baliyan, R. Mukherjee, A. Priyadarshini, A. Vibhuti, A. Gupta, R. P. Pandey, and C. M. Chang, “Determination of antioxidants by DPPH radical scavenging activity and quantitative phytochemical analysis of Ficus religiosa,” Molecules, vol. 27, no. 4, 2022, https://doi.org/10.3390/molecules27041326.
N. Tena, J. Martín, and A. G. Asuero, “State of the art of anthocyanins: Antioxidant activity, sources, bioavailability, and therapeutic effect in human health,” Antioxidants, vol. 9, no. 5, p. 451, 2020, https://doi.org/10.3390/antiox9050451.
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Rinten Anjang Sari

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Authors who publish with the Journal of Agri-food Science and Technology (JAFOST) agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License (CC BY-SA 4.0) that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial publication in this journal.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work.



