Effect of Sintering Temperature on Crystal Structure and Conductivity of the CaCO3-Doped Li4Ti5O12 Anodes from Blood Clam Shells (Anadara granosa)
DOI:
https://doi.org/10.12928/irip.v5i1.4804Keywords:
Blood Clam Shell, Conductivity, Li4Ti5O12, Sintering TemperatureAbstract
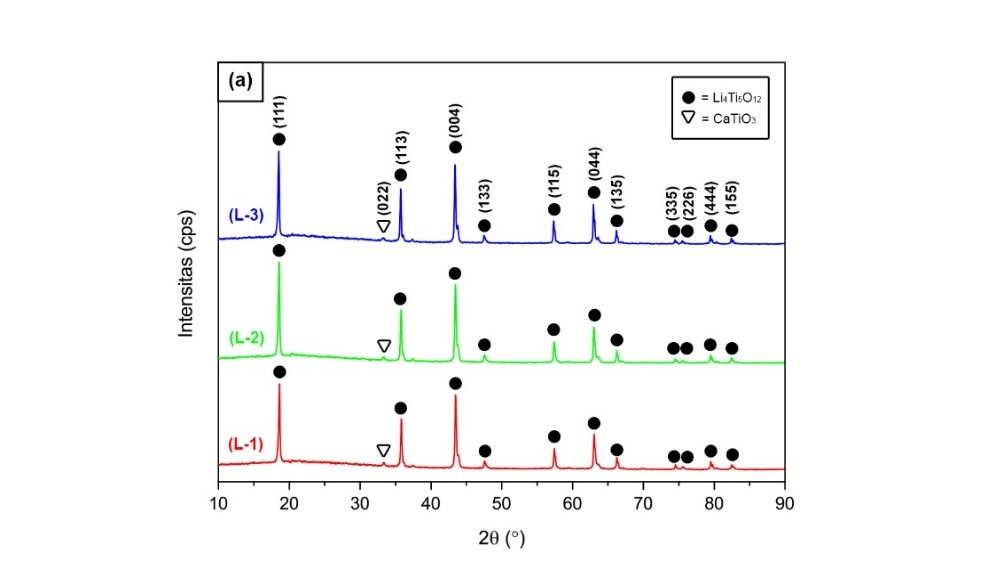
CaCO3-doped Li4Ti5O12 was synthesized by solid-state method with sintering temperatures at 750 °C, 800 °C, and 850 °C. The source of CaCO3 was used from blood clam shells (Anadara granosa) with a content of 97.67%. The influence of sintering temperature on crystal structure and conductivity of CaCO3-doped Li4Ti5O12 are extensively studied. XRD results show there is no CaCO3 phase found, which indicates that the doping of Li4Ti5O12 with CaCO3 has been successful. The smallest crystallite size was obtained at a sintering temperature of 800 °C, which is 46.49 nm, which is beneficial for shortening diffusion length and facilitating the electron and ion transport, causing an increase in anode conductivity. The most optimal conductivity was obtained in samples with a sintering temperature of 800 °C with a conductivity of 2.46 x 10-4 S/cm. When the sintering temperature is increased to 850 °C, the particles tend to agglomerate and deteriorate the electrochemical properties.
References
H. Yan, D. Zhang, Qilu, X. Duo, and X. Sheng, “A Review of Spinel Lithium Titanate (Li4Ti5O12) as Electrode Material for Advanced Energy Storage Devices,” Ceram. Int., Vol. 47, No. 5, pp. 5870–5895, 2021, Doi: 10.1016/j.ceramint.2020.10.241.
B. Tian, H. Xiang, L. Zhang, Z. Li, and H. Wang, “Niobium Doped Lithium Titanate as a High Rate Anode Material for Li-Ion Batteries,” Electrochim. Acta, Vol. 55, No. 19, pp. 5453–5458, 2010, Doi: 10.1016/j.electacta.2010.04.068.
A. Subhan, D. Setiawan, and S. A. Saptari, “The 15th International Conference Quality In Research (QiR) 2017 Preparation and Ionic Conductivity of Li3.9Ca0.1Ti5o12 Using Waste Chicken Eggshells as Ca Source for Anode Material of Lithium-Ion Batteries,” IOP Conf. Ser. Mater. Sci. Eng., Vol. 316, No. 1, pp. 0–10, 2018, Doi: 10.1088/1757-899X/316/1/012048.
T. P. Zhou, X. Y. Feng, X. Guo, W. W. Wu, S. Cheng, and H. F. Xiang, “Solid-State Synthesis and Electrochemical Performance of Ce-Doped Li4Ti5O12 Anode Materials for Lithium-Ion Batteries,” Electrochim. Acta, Vol. 174, pp. 369–375, 2015, Doi: 10.1016/j.electacta.2015.06.021.
X. Guo et al., “Solid-State Synthesis and Electrochemical Performance of Li 4Ti5O12/Graphene Composite for Lithium-Ion Batteries,” Electrochim. Acta, Vol. 109, pp. 33–38, 2013, Doi: 10.1016/j.electacta.2013.07.058.
Q. Zhang, M. G. Verde, J. K. Seo, X. Li, And Y. S. Meng, “Structural and Electrochemical Properties of Gd-Doped Li4Ti5O12 as Anode Material with Improved Rate Capability for Lithium-Ion Batteries,” J. Power Sources, Vol. 280, pp. 355–362, 2015, Doi: 10.1016/j.jpowsour.2015.01.124.
L. Cheng, J. Yan, G. N. Zhu, J. Y. Luo, C. X. Wang, and Y. Y. Xia, “General Synthesis of Carbon-Coated Nanostructure Li4Ti5O12 as a High Rate Electrode Material for Li-Ion Intercalation,” J. Mater. Chem., Vol. 20, No. 3, pp. 595–602, 2010, Doi: 10.1039/B914604K.
H. Park, T. Song, H. Han, and U. Paik, “Electrospun Li4Ti5O12 Nanofibers Sheathed with Conductive Tin/Tioxny Layer as an Anode Material for High Power Li-Ion Batteries,” J. Power Sources, Vol. 244, pp. 726–730, 2013, Doi: 10.1016/j.jpowsour.2012.11.078.
M. Guo, S. Wang, L.-X. Ding, C. Huang, and H. Wang, “Tantalum-doped Lithium Titanate with Enhanced Performance for Lithium-ion Batteries,” J. Power Sources, vol. 283, pp. 372–380, Jun. 2015, doi: 10.1016/j.jpowsour.2015.02.154.
B. Wang, J. Wang, J. Cao, H. Ge, and Y. Tang, “Nitrogen-Doped Li4Ti5O12 Nanosheets With Enhanced Lithium Storage Properties,” J. Power Sources, Vol. 266, pp. 150–154, 2014, Doi: 10.1016/j.jpowsour.2014.05.009.
Y. Qi, Y. Huang, D. Jia, S. J. Bao, and Z. P. Guo, “Preparation and Characterization of Novel Spinel Li4Ti5O12-XBrx Anode Materials,” Electrochim. Acta, Vol. 54, No. 21, pp. 4772–4776, 2009, Doi: 10.1016/j.electacta.2009.04.010.
S. Huang, Z. Wen, X. Zhu, and Z. Gu, “Preparation and Electrochemical Performance of Ag Doped Li4Ti5O12,” Electrochem. Commun., Vol. 6, No. 11, pp. 1093–1097, 2004, Doi: 10.1016/j.elecom.2004.08.013.
Q. Zhang, C. Zhang, B. Li, S. Kang, X. Li, and Y. Wang, “Preparation and Electrochemical Properties of Ca-Doped Li4Ti5O12 as Anode Materials in Lithium-Ion Battery,” Electrochim. Acta, Vol. 98, pp. 146–152, 2013, Doi: 10.1016/j.electacta.2013.03.006.
X. Deng, W. Li, M. Zhu, D. Xiong, and M. He, “Synthesis of Cu-Doped Li4Ti5O12 Anode Materials With a Porous Structure for Advanced Electrochemical Energy Storage: Lithium-Ion Batteries,” Solid State Ionics, Vol. 364, No. March, pp. 115614, 2021, Doi: 10.1016/j.ssi.2021.115614.
L. Hou, X. Qin, X. Gao, T. Guo, X. Li, and J. Li, “Zr-Doped Li4Ti5O12 Anode Materials With High Specific Capacity for Lithium-Ion Batteries,” J. Alloys Compd., Vol. 774, pp. 38–45, 2019, Doi: 10.1016/j.jallcom.2018.09.364.
X. Bai, W. Li, A. Wei, Q. Chang, L. Zhang, and Z. Liu, “Preparation and Electrochemical Performance of F-Doped Li4Ti5O12 For Use in the Lithium-Ion Batteries,” Solid State Ionics, Vol. 324, No. April, pp. 13–19, 2018, Doi: 10.1016/j.ssi.2018.06.005.
B. Priyono, D. K. Ibrahimi, A. Z. Syahrial, H. Jodi, A. Subhan, and M. R. Nugraha, “Synthesis and Characterization of Ca-Doped Li4Ti5O12 Using CaCo3 from Chicken Eggshell as a Dopant for Lithium-Ion Battery Anode Material,” IOP Conf. Ser. Mater. Sci. Eng., Vol. 547, No. 1, pp. 0–12, 2019, Doi: 10.1088/1757-899X/547/1/012040.
P. M. Insani S and R. Rahmatsyah, “Analisis Pola Struktur Kalsium Karbonat (CaCO3) pada Cangkang Kerang Darah (Anadara Granosa) di Bukit Kerang Kabupaten Aceh Tamiang [Analysis of Structural Patterns of Calcium Carbonate (CaCO3) in Blood Shells (Anadara Granosa) in Bukit Kerang, Aceh Tamiang Regency],” J. Teor. dan Apl. Fis., Vol. 9, No. 1, pp. 23–32, 2021, Doi: 10.23960/jtaf.v9i1.2717.
C. Y. Lin and J. G. Duh, “Porous Li4Ti5O12 Anode Material Synthesized By One-Step Solid-State Method for Electrochemical Properties Enhancement,” J. Alloys Compd., Vol. 509, No. 8, pp. 3682–3685, 2011, Doi: 10.1016/j.jallcom.2010.12.160.
C. Qiu, Z. Yuan, L. Liu, N. Ye, and J. Liu, “Sol-Gel Preparation and Electrochemical Properties of La-Doped Li4Ti5O12 Anode Material for Lithium-Ion Battery,” J. Solid State Electrochem., Vol. 17, No. 3, pp. 841–847, 2013, Doi: 10.1007/s10008-012-1930-1.
J. Y. Lin, C. C. Hsu, H. P. Ho, And S. H. Wu, “Sol-Gel Synthesis Of Aluminum Doped Lithium Titanate Anode Material For Lithium-Ion Batteries,” Electrochim. Acta, Vol. 87, pp. 126–132, 2013, Doi: 10.1016/j.electacta.2012.08.128.
P. S. Yin, H. T. Peng, Y. Xiao, T. W. Lin, and J. Y. Lin, “Facile Synthesis of an Al-doped carbon-coated Li4Ti5O12 Anode for High-Rate Lithium-Ion Batteries,” Rsc Adv., Vol. 6, No. 81, pp. 77151–77160, 2016, Doi: 10.1039/C6RA11353B.
V. D. Nithya, R. Kalai Selvan, K. Vediappan, S. Sharmila, and C. W. Lee, “Molten Salt Synthesis and Characterization of Li4Ti5-xMnxO12 (x = 0.0, 0.05 and 0.1) as Anodes for Li-Ion Batteries,” Appl. Surf. Sci., Vol. 261, pp. 515–519, 2012, Doi: 10.1016/j.apsusc.2012.08.047.
Y. Ge et al., “Copper-Doped Li4Ti5O12/Carbon Nanofiber Composites as Anode for High-Performance Sodium-Ion Batteries,” J. Power Sources, Vol. 272, pp. 860–865, 2014, Doi: 10.1016/j.jpowsour.2014.08.131.
S. Abureden et al., “Multigrain Electrospun Nickel Doped Lithium Titanate Nanofibers With High Power Lithium-Ion Storage,” J. Mater. Chem. A Vol. 4, No. 32, pp. 12638–12647, 2016, Doi: 10.1039/C6TA04046B.
F. Zhao, P. Xue, H. Ge, L. Li, and B. Wang, “ Na-Doped Li4Ti5O12 as an Anode Material for Sodium-Ion Battery With Superior Rate and Cycling Performance,” J. Electrochem. Soc., Vol. 163, No. 5, pp. A690–A695, 2016, Doi: 10.1149/2.0781605jes.
L. Wang et al., “Structural and Electrochemical Characteristics of Ca-Doped ‘Flower-Like’ Li4Ti5O12 Motifs as High-Rate Anode Materials for Lithium-Ion Batteries,” Chem. Mater., Vol. 30, No. 3, pp. 671–684, 2018, Doi: 10.1021/acs.chemmater.7b03847.
T. F. Yi, S. Y. Yang, X. Y. Li, J. H. Yao, Y. R. Zhu, and R. S. Zhu, “Sub-Micrometric Li4-xNaxTi5O12 (0 ≤ X ≤ 0.2) Spinel as Anode Material Exhibiting High Rate Capability,” J. Power Sources, Vol. 246, pp. 505–511, 2014, Doi: 10.1016/j.jpowsour.2013.08.005.
H. Deng et al., “High Rate Performance of Ca-Doped Li4Ti5O12 Anode Nanomaterial for the Lithium-Ion Batteries,” J. Nanomater., Vol. 2018, pp. 1–7, 2018, Doi: 10.1155/2018/7074824.
T. Masindi and N. Herdyastuti, “Karakterisasi Kitosan dari Cangkang Kerang Darah (Anadara Granosa) [Characterization Chitosan from the Shells of Blood Clams (Anadara Granosa)],” J. Chem., vol. 6, no. 3, pp. 137–142, 2017, doi: 10.26740/ujc.v6n3.p%25p.
G. K. Saputra, Evahelda, and E. Bidayani, “Faktor-Faktor Sosial Ekonomi yang Mempengaruhi Usaha Budidaya Kerang Darah (Anadara Granosa) di Kabupaten Bangka Barat [Socio-Economic Factors Affecting Blood Shellfish (Anadara Granosa) Cultivation Business in West Bangka Regency],” J. Integr. Agribus., Vol. 1, No. 2, pp. 67–81, 2019, doi: 10.33019/jia.v1i2.1017.
Nurhayati, Muhdarina, A. Linggawati, S. Anita, and T. A. Amri, “Preparation and Characterization of Calcium Oxide Heterogeneous Catalyst Derived from Anadara Granosa Shell for Biodiesel Synthesis,” KnE Eng., vol. 1, Sep. 2016, Doi: 10.18502/keg.v1i1.494.
S. N. F. Moideen Et Al., “Wasted Cockle Shell (Anadara Granosa) as a Natural Adsorbent for Treating Polluted River Water in the Fabricated Column Model (FCM),” Desalin. Water Treat., Vol. 57, No. 35, pp. 16395–16403, 2016, Doi: 10.1080/19443994.2015.1082939.
K. Dhanaraj and G. Suresh, “Conversion of Waste Sea Shell (Anadara Granosa) Into Valuable Nanohydroxyapatite (Nhap) for Biomedical Applications,” Vacuum, Vol. 152, pp. 222–230, 2018, Doi: 10.1016/j.vacuum.2018.03.021.
X. Zhang et al., “Influence of Sintering Temperature and Graphene Additives on the Electrochemical Performance of Porous Li4Ti5O12 Anode for Lithium-Ion Capacitor,” Electrochim. Acta, Vol. 246, pp. 1237–1247, 2017, Doi: 10.1016/j.electacta.2017.07.014.
W. R. M. Saka, “Pengaruh Temperatur Hidrotermal Pada Proses Sintesis Li4Ti5O12 Nanowire Terhadap Performa Elektrokimia [Effect of Hydrothermal Temperature on Li4Ti5O12 Nanowire Synthesis Process on Electrochemical Performance],” Institut Teknologi Sepuluh Nopember, 2016.
H. Zhang, Y. Yang, H. Xu, L. Wang, X. Lu, and X. He, “Li4Ti5O12 Spinel Anode: Fundamentals and Advances in Rechargeable Batteries,” Infomat, Vol. 4, No. 4, pp. 1–29, 2022, Doi: 10.1002/inf2.12228.
F. Akbar et al., “Sintesis Ca2P2O7 dari Limbah Kerang sebagai Bahan Baku Limbah Cangkang Kerang dengan Metode Solvothermal [Synthesis of Ca2P2O7 from Shellfish Waste as Raw Material for Shellfish Shell Waste by Solvothermal Method],” J. Fis. Dan Apl., Vol. 15, No. 3, pp. 110, 2019, Doi: 10.12962/j24604682.v15i3.4707.
K. N. Wahyusi, N. Karunia, and M. Satrya, “Precipitation Method in Calcium Phosphate Synthesis from Blood Clamshells (Anadara Granosa),” J. Phys. Conf. Ser., Vol. 1899, No. 1, 2021, Doi: 10.1088/1742-6596/1899/1/012057.
H. Bharatham, M. Z. A. B. Zakaria, E. K. Perimal, L. M. Yusof, and M. Hamid, “Mineral and Physiochemical Evaluation of Cockle Shell (Anadara granosa) and Other Selected Molluscan Shell as Potential Biomaterials,” Sains Malaysiana, Vol. 43, No. 7, pp. 1023–1029, 2014.
A. W. Harahap, Z. Helwani, Z. Zultiniar, and Y. Yelmida, “Sintesis Hidroksiapatit Melalui Precipitated Calcium Carbonate (PCC) Cangkang Kerang Darah dengan Metode Hidrotermal pada Variasi pH dan Waktu Reaksi [Synthesis of Hydroxyapatite Through Precipitated Calcium Carbonate (PCC) Blood Shells with Hydrothermal Methods at Variations in pH and Reaction Time],” Jom FTEKNIK, vol. 2, no. 2, pp. 1–8, 2015, [Online]. Available: https://jom.unri.ac.id/index.php/JOMFTEKNIK/article/view/8154.
H. S. Bhatti, S. Jabeen, A. Mumtaz, G. Ali, S. Qaisar, and S. Hussain, “Effects of Cobalt Doping on Structural, Optical, Electrical and Electrochemical Properties of Li4Ti5O12 Anode,” J. Alloys Compd., Vol. 890, pp. 161691, 2022, Doi: 10.1016/j.jallcom.2021.161691.
M. Harun-Or-Rashid, M. N. Islam, M. Arifuzzaman, and A. K. M. A. Hossain, “Effect of Sintering Temperature on the Structural, Morphological, Electrical, and Magnetic Properties of Ni–Cu–Zn And Ni–Cu–Zn–Sc Ferrites,” J. Mater. Sci. Mater. Electron., Vol. 32, No. 2, pp. 2505–2523, 2021, Doi: 10.1007/s10854-020-05018-7.
H. Rofiko, Y. Iriani, and R. Suryana, “Pengaruh Suhu Sintering pada Pembuatan Strontium Titanat (SrTiO3) Terhadap Konstanta Dielektrik Menggunakan Metode Co-Precipitation [Effect of Sintering Temperature on the Preparation of Strontium Titanate (SrTiO3) on Dielectric Constant Using Co-Precipitation Method],” Indones. J. Appl. Phys., Vol. 7, No. 1, pp. 27, 2017, Doi: 10.13057/ijap.v7i1.1778.
S. uwarni, A. Zaidah, A. Supriyanto, A. Jamaluddin, and Y. Iriani, “Struktur Mikro dan Sifat Listrik Material Ferroelektrik Barium Titanat dengan doping Stronsium [Microstructure and Electrical Properties of Ferroelectric Materials Barium Titanate with Strontium Doping],” J. Fis. dan Apl., vol. 11, no. 3, pp. 99, Oct. 2015, doi: 10.12962/j24604682.v11i3.1067.
X.-C. Zhao Et Al., “ Hydrothermally Synthesized Li4Ti5O12 Nanotubes Anode Material with Enhanced Li-Ion Battery Performances,” J. Nanosci. Nanotechnol., Vol. 19, No. 11, pp. 7387–7391, 2019, Doi: 10.1166/jnn.2019.16668.
A. Bouhamed, A. Al-Hamry, C. Müller, S. Choura, and O. Kanoun, “Assessing the Electrical Behaviour of MWCNTs/Epoxy Nanocomposite for Strain Sensing,” Compos. Part B Eng., Vol. 128, pp. 91–99, 2017, Doi: 10.1016/j.compositesb.2017.07.005.
Downloads
Published
Issue
Section
License
Copyright (c) 2022 Marhan Ebit Saputra, Megawati Ayu Putri, Eka Febrianti, Widodo Budi Kurniawan

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Authors who publish in IRiP agree to the following terms: Authors retain copyright and grant the IRiP right of first publication with the work simultaneously licensed under a Creative Commons Attribution License (CC BY-SA 4.0) that allows others to share (copy and redistribute the material in any medium or format) and adapt (remix, transform, and build upon the material) the work for any purpose, even commercially with an acknowledgment of the work's authorship and initial publication in IRiP. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial publication in IRiP. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See The Effect of Open Access).














