ISSN: 2685-9572 Buletin Ilmiah Sarjana Teknik Elektro
Vol. 8, No. 1, February 2026, pp. 64-84
A Systematic Review of Machine Learning and Deep Learning Approaches in MRI-Based Brain Tumour Analysis, Detection and Classification
Hanan M. Omran 1, Khalil Ibrahim 2,3, Gamal T. Abdel-Jaber 1, 3, Abdel-Nasser Sharkawy 1,4
1 Mechanical Engineering Department, Faculty of Engineering, Qena University, Qena 83523, Egypt
2 Mechatronics Engineering Department, Faculty of Engineering, Assiut University, Egypt
3 New Assiut Technological University (NATU), New Assiut City, Assiut, 71684, Egypt
4 Mechanical Engineering Department, College of Engineering, Fahad Bin Sultan University, Tabuk 47721, Saudi Arabia
ARTICLE INFORMATION |
| ABSTRACT |
Article History: Received 08 September 2025 Revised 23 December 2025 Accepted 13 January 2026 |
| 
A brain tumour develops when abnormal cell growth happens in or near the brain. These tumours can grow slowly and not be cancerous, or they can grow quickly and spread, which is known as malignancy. Brain tumours put pressure on the surrounding brain tissues, causing symptoms like memory loss, migraines, movement dysfunction, and vision impairment. Brain tumours are often divided into two groups: primary tumours, which start in the brain, and secondary tumours, which are caused by cancers that spread to other regions of the body. Although brain tumours provide a significant medical challenge, patient outcomes have improved thanks to recent advancements in diagnostic and treatment methods. Because of its better soft-tissue contrast and noninvasive nature, magnetic resonance imaging (MRI) is one of the most important medical imaging modalities for the early identification and precise localization of brain tumours. Clinical practice also makes use of other imaging methods such as PET-CT and functional MRI (fMRI). Artificial intelligence and deep learning techniques have demonstrated significant promise in automated brain cancer analysis in recent years. These methods enable precise cancer diagnosis, classification, and segmentation by identifying intricate patterns from MRI data that are challenging to recognize through manual examination. A thorough study of current deep learning and machine learning techniques for MRI-based brain tumour analysis is provided in this paper. The current thorough literature search includes papers released between 2019 and 2024. 67 pertinent articles are chosen for in-depth analysis after predetermined inclusion and exclusion criteria is used. Many of these studies make use of publicly accessible datasets like Figshare, TCIA, and BraTS. The results show that deep learning models frequently outperform traditional machine learning methods in terms of accuracy and robustness, especially convolutional neural network-based designs. However, there are still issues with clinical generalisation, model interpretability, and data heterogeneity. |
Keywords: Brain Tumour Analysis; Tumour Detection and Classification; Magnetic Resonance Imaging (MRI); Machine Learning Approaches; Deep Learning Models; Convolutional Neural Networks (CNNs); Systematic Review |
Corresponding Author: Abdel-Nasser Sharkawy, Mechanical Engineering Department, Faculty of Engineering, Qena University, Qena 83523, Egypt. Email: abdelnassersharkawy@eng.svu.edu.eg |
This work is open access under a Creative Commons Attribution-Share Alike 4.0 
|
Document Citation: M. M. Omran, K. Ibrahim, G. T. Abdel-Jaber, and A. -N. Sharkawy, “A Systematic Review of Machine Learning and Deep Learning Approaches in MRI-Based Brain Tumour Analysis, Detection and Classification,” Buletin Ilmiah Sarjana Teknik Elektro, vol. 8, no. 1, pp. 64-84, 2026, DOI: 10.12928/biste.v8i1.14673. |
- INTRODUCTION
The brain, which is the most complex organ in the human body, oversees numerous essential functions and cognitive tasks such as reasoning, decision-making, coordinating both motor and sensory activities, and relaying messages to the rest of the body via nerve signals [1][2]. It is susceptible to several illnesses, including brain tumors, which are the second leading cause of death after heart disease, due to its anatomical and functional complexity [3][4]. Uncontrolled proliferation that results in the formation of a tumor mass is caused by aberrant cell growth that does not follow the regular life cycle, which consists of growth followed by programmed cell death, or apoptosis [5]-[8]. Tumors are frequently categorized based on the organ or tissue from which they originate. Therefore, tumors that develop in any part of the brain or skull, including tissues, membranes, nerves, and bones, are classified as brain tumors and account for up to 70% of cases, which is a high death rate [9].
According to worldwide data from Cancer.Net, in 2020, over 308,102 new cases were identified as primary brain or spinal cord tumors, and about 251,329 individuals died from malignant brain and central nervous system (CNS) tumors [10]-[13]. Based on where they originate, brain tumors are categorized into two primary types: primary tumors, which are made up of brain cells, like gliomas and meningiomas, and secondary or metastatic tumors, which develop when cancer cells from other organs, like the breast or lung, spread and end up in the brain [14][15]. Only around 2% of the 250,000 brain tumors identified each year worldwide are categorized as malignant, according to other reports [16]. The importance of brain tumors to public health is demonstrated by these numbers, which also emphasize the necessity of creating precise and efficient diagnostic and therapeutic approaches based on artificial intelligence and contemporary medical imaging technology.
Brain tumors are very different and hard to treat, which makes them one of the toughest problems in medical care. To get a correct diagnosis, several important steps must be done. These include finding the tumor first, then dividing the image to clearly see where the tumor starts and ends, and finally figuring out what type and how severe the tumor is. Each of these steps is considered very important for better patient care and making a good treatment plan [17]-[20]. There are several methods used to look at the brain, such as computed tomography (CT), single-photon emission computed tomography (SPECT), and positron emission tomography (PET). These help in checking how the brain is working and its structure. However, the most commonly used method is magnetic resonance imaging (MRI), because it doesn't use harmful radiation and gives very clear images of the body's tissues [21]-[23]. Figure 1 shows the process for analyzing brain tumors.
A brain MRI scan uses multi-planar imaging to collect three-dimensional data, showing the brain from three main directions: coronal, axial, and sagittal. This lets doctors see the brain's structure clearly from different angles with high detail. The quality of the images, like how thick each slice is and the space between slices, depends on the techniques used to collect data and the strength of the magnetic field during the scan [24][25]. In NMR imaging, different image sequences are made by changing the timing between signals that happen at various radio frequencies. The most common sequences are T1-weighted, T2-weighted, and FLAIR (Fluid-Attenuated Inversion Recovery). These sequences are important because they give detailed information about the inside of the brain and help doctors tell the difference between tumors, normal tissue, and fluid [26]. Every sequence illustrates a distinct area of the brain or skull, providing essential insights into a tumor's behavior, its location, and its appearance. Medical visualization plays an important role in taking into account brain tumors. It helps doctors to improve their diagnosis and discover the best ways to treat them. This process creates several steps. First, use MRI and special IT tools to find areas where the tumor may be present. Detection of these regions is an important first step [27]-[29]. Next, segmentation is used to clearly indicate the size and edge of the tumor. This will help the physician to determine whether surgery or radiation is required and how far the tumor will spread [30]. This classification will then help physicians understand which tumors are, such as gliomas and meningiomas, and how serious it is to the cells involved [31]-[33]. Finally, they make predictions in how the tumor will work and how it can respond to a variety of treatment options. This helps in creating a personalized treatment plan that protects the confidentiality of each patient [34]-[36]. All of these steps help diagnose and treat more accurately and efficiently, leading to the best results related to the patient's health.
Brain tumors are generally estimated by radiologists who visually examine MRI, a standard clinical procedure. However, this method is generally complicated and unrealistic about the large number of images of patients, the possibility of human error caused by fatigue, differences in interpretation between x-rays, and the differences in many cases. This results in the process being errors and very long [21]. Furthermore, manual distinction between tumor edges can lead to significant changes in the outcome, which can affect radiological estimations and lead to inconsistent results. It should be noted that tumor types have a significant impact on the accuracy of the concentration learning model. For example, Glioblastoma Multiforme (GBM) and other aggressive gliomas provide considerable difficulties because their dispersed nature and inconsistent boundaries complicate the segmentation process and drastically lower accuracy [37]. Conversely, clearly defined solid tumors lead to increased accuracy rates because of their easier identification within images. Doctors' ability to accurately detect disease decreases when faced with low contrast or images affected by interference, noise, and artifacts, opening an opportunity for deep learning algorithms to be more effective. With proper training using varied and superior datasets, these algorithms can identify subtle indicators and patterns that might be overlooked by human professionals. Investigators have created enhancement techniques such as attention-driven frameworks, preprocessing procedures, and training approaches that are resistant to artifacts to ensure consistent and trustworthy performance, because image inconsistencies and disruptions can negatively affect model effectiveness [38].
The swift escalation in worldwide data aggregation, coupled with the progress of deep learning methodologies, has triggered a significant transformation in how brain tumors are diagnosed and categorized via magnetic resonance imaging (MRI), thereby prompting an extreme advancement in the domain of medical image manipulation recently. Historically, assessments of tumors were conducted using standard qualitative benchmarks, encompassing factors such as the integrity of tissue connection with adjacent tissue, tumor density, and the clarity of cellular structure within the impacted zone. Currently, it has become achievable to execute accurate quantitative evaluations pertaining to the histological and neuropathological attributes of tumors, which includes ascertaining their dimensions, configuration, and textural arrangements, all as a consequence of technological enhancements [39]. The domains of artificial intelligence (AI) and computer vision have realized noteworthy progress in the investigation of brain tumors, thereby amplifying the precision and effectiveness of diagnostic processes, which has unlocked possibilities for cutting-edge personalized medical implementations, as well as leading to more favorable treatment results [40][41].
More and more, scientists are utilizing artificial intelligence (AI) approaches, specifically machine learning (ML) and deep learning (DL), to improve or computerize diagnosis procedures and overcome the challenges associated with diagnosing brain tumors by hand. Despite their initial usefulness, conventional AI methods like rule-based expert systems have considerable shortcomings in their ability to generalize because of the complicated and varied properties of brain tumors [42]. Machine learning methods like k-nearest neighbours (k-NN), random forests (RF), and support vector machines (SVMs) have helped classify tumors using data from magnetic resonance imaging (MRI) or radiomic signals [43]. But these methods depend a lot on features that are created by people, which limits how well they can handle new types of images or patterns [44].
Deep learning, especially convolutional neural networks (CNNs), has changed how we look at brain tumors. CNNs are special because they can learn complicated features directly from raw data, without needing people to design them. This makes them better at tasks like finding, dividing, and sorting tumors [45]. This new way of doing things has replaced old manual methods that made it hard to use these models widely. As technology moves forward, models now use transformer designs and attention mechanisms, which help them do better, especially when understanding how different features relate to each other in space [46][47]. Another big benefit is that these models can handle large MRI datasets, giving reliable and consistent results that help doctors make decisions and reduce differences in how people evaluate things [48]. Figure 2 shows how deep learning compares to manual methods in diagnosing brain tumors.
This review aims to enhance the scientific knowledge of brain tumors through discussing their important aspects. The work presents a comprehensive analysis of current deep learning and machine learning techniques employed in brain tumor research, focusing on detection, segmentation, and classification as key applications. The investigation also looks into the diverse methodologies incorporated within these models, the datasets used to evaluate their performance, and the benchmarks applied to measure their effectiveness. The study's summary features a concise overview of the main results and suggestions for future studies designed to increase the clinical applicability, precision, and performance of existing models.
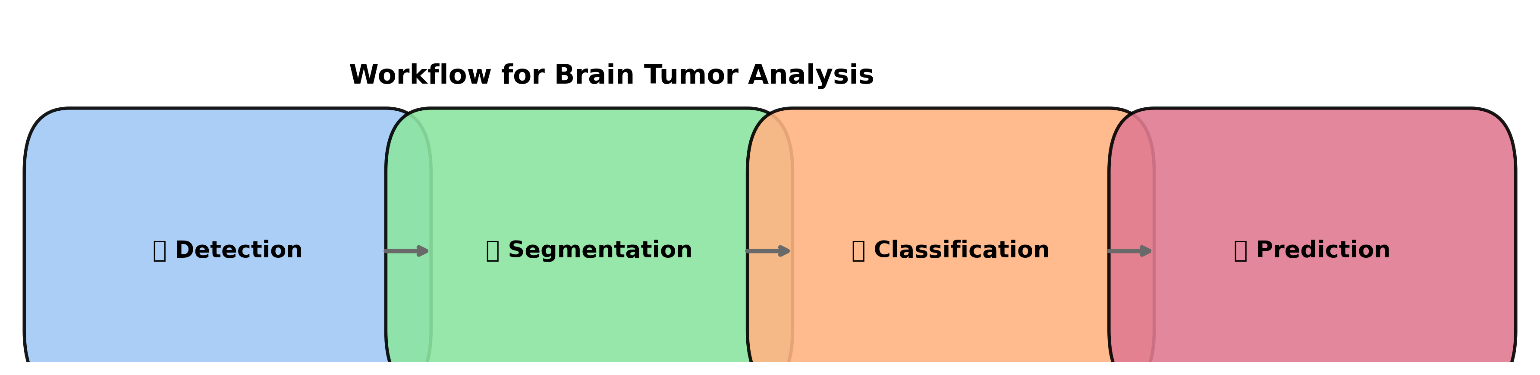
Figure 1. Workflow for Brain Tumor Analysis
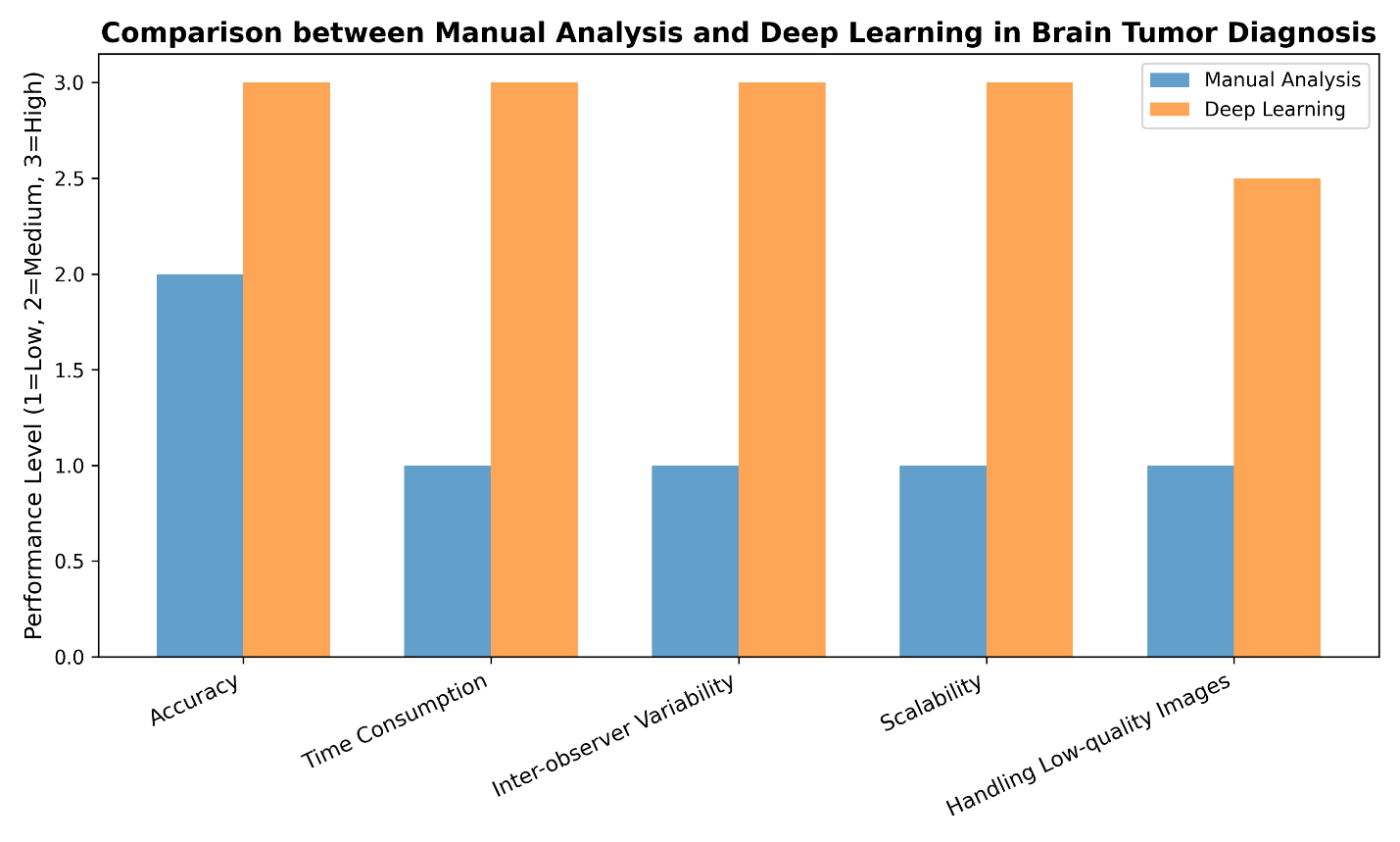
Figure 2. Comparison between Manual Analysis and Deep Learning in Brain Tumor Diagnosis
- PROPOSED METHODOLOGY
The approach used to carry out this systematic review is explained in this section. In order to find and examine pertinent research articles on MRI-based brain tumour analysis utilising machine learning and deep learning techniques, the entire approach consists of a literature search strategy, study selection criteria, and data extraction procedure.
- Review Plan
This study uses a systematic review method to find, look at, and bring together recent research on machine learning and deep learning techniques for detecting, separating, and categorizing brain tumors using MRI data. The plan for the review was made following the PRISMA guidelines, which help make the process clear, repeatable, and scientifically strong.
- Data Sources and Search Strategy
A thorough literature search was carried out using different databases of engineering, medical imaging, and artificial intelligence researches. To guarantee that the evaluation includes the most recent developments in machine learning and deep learning approaches, the search was restricted to research published between January 2019 and December 2024. Boolean operators were used in the search method to integrate keywords associated with brain tumours, MRI imaging, and artificial intelligence. The following is how the primary search string was created: "Brain Tumour" or "Brain Neoplasm," "MRI" or "Magnetic Resonance Imaging," and "Machine Learning" or "Deep Learning" or "Convolutional Neural Network" or "CNN" Only English-language conference papers and peer-reviewed journal publications were taken into account.
- Inclusion and Exclusion Criteria
The collected studies were vetted using predetermined inclusion and exclusion criteria to guarantee quality and relevance as follows.
- Criteria for inclusion
The papers included in the presented systematic literature review are selected based on the criteria of inclusion as pointed in the following points.
- Research released between 2019 and 2024.
- Articles about brain tumour analysis with MRI.
- Utilizing deep learning or machine learning models.
- Research that focusses on at least one task, such as classification, segmentation, or detection.
- Experimental results with quantitative evaluation measures are available.
- Criteria for exclusion
The papers excluded in the presented systematic literature review are based on the criteria of exclusion as pointed in the following points.
- Research unrelated to magnetic resonance imaging.
- Articles on tumours outside of the brain.
- Short abstracts, editorials, and review pieces.
- Research without experimental support.
- Extended versions of the same work or duplicate publications.
- Study Selection Process
There were several steps in the selecting procedure for the study. In order to eliminate unnecessary research, all obtained records were first reviewed using titles and abstracts. The remaining articles' full texts were evaluated in the second step in relation to the inclusion and exclusion criteria. Duplicate studies were found and eliminated. 67 original research publications in all were chosen for final analysis following the screening procedure. A PRISMA flow diagram, which shows the quantity of records found, screened, eliminated, and included in the final review, summarizes the entire selection process.
- Data Extraction and Analysis
The following data was methodically gathered from each chosen study or paper:
- Year of publication.
- Utilized datasets (such as BraTS, Figshare, and private datasets).
- T1, T2, FLAIR, and other MRI sequences.
- Deep learning or machine learning models.
- Target task (classification, segmentation, or detection).
- Evaluation metrics (F1-score, accuracy, precision, recall, and dice score).
To find architectural themes, performance patterns, and common issues across experiments, the retrieved data was qualitatively analyzed. A quantitative meta-analysis was not carried out due to the variability of datasets and evaluation procedures.
- Limitations of the Review
Despite the rigorous methods used in this review, there are limitations still exist. Many relevant studies (more than 67 articles) can be considered in future work for deep analysis and investigations. Additionally, the direct comparability of published results may be impacted by variations in datasets, evaluation measures, and experimental setups among investigations.
- BRAIN TUMORS ANALYSIS
Currently, machine learning techniques are applied within medical imaging for effectively interpreting intricate information, thus establishing their usefulness. The utilization of machine learning is particularly important in the examination of brain tumors through magnetic resonance imaging (MRI) because it defines a system's capability to learn through data and utilize what it has learned when analyzing new information without explicit human direction [49].The machine learning system aids in the sorting of brain images, the prediction of tumor presence, and the assistance of treatment decisions by identifying patterns and extracting features [50]. Generalization, the primary objective of certain machine learning algorithms, is to produce accurate predictions on fresh data that differs from the data that was used for training. To achieve this, the model has to undergo modification and refinement using the data it was trained on, which ensures the precision of future forecasts [51].
Various essential machine learning methods are widely utilized in the field of medical imaging, such as k-nearest neighbours (KNNs), feed-forward neural networks (FNNs), artificial neural networks (ANNs), backpropagation neural networks (BPNNs), as well as support vector machines (SVMs) [52]. According to [52], these techniques are employed to enhance MRI image analysis and speed up and increase the accuracy of brain tumor diagnosis. Advantages and disadvantages of common machine learning algorithms (SVM, ANN, BPNN, KNN) applied in brain tumor analysis are shown in Figure 3, where the model learns from training data and is then assessed and refined to be able to make accurate predictions when faced with new, unknown data [53]. These approaches, however, have some drawbacks, chief among them the requirement for sizable, meticulously annotated data sets and the variation in imaging processes throughout facilities, which could compromise the reliability and interpretability of the findings in clinical settings [54].
In many tasks, deep learning has surpassed conventional algorithms in recent years, making it a sophisticated subfield of machine learning (ML). Deep learning models possess the capability to acquire feature hierarchies directly from unprocessed information, distinguishing themselves from traditional methods reliant on handcrafted features, and potentially leading to superior performance across diverse applications [44]. Within the realm of medical applications, convolutional neural networks (CNNs) stand out as a prominent and frequently employed deep learning technique. Their widespread adoption in medical image analysis stems from their proficiency in autonomously handling extensive image datasets and discerning complex patterns such as forms, lines, and surface characteristics [55].
Deep learning techniques have brought about a revolution in how brain tumors are analyzed using magnetic resonance imaging scans. They've eliminated the need to manually identify and select key attributes, which has enhanced both the precision and effectiveness of the analysis. Typically, this process starts with obtaining the images and performing initial refinements to boost image quality and prepare them for subsequent examination. After these improvements, exquisite images are introduced into deeper training structures such as neuronal networks, as shown in Figure 4. This structure extracts features in an automated way and handles specific features manually. Next, the validity of the model is evaluated to ensure the accuracy and reliability of brain tumor analysis using MRI analysis, as shown in Figure 5. This approach is used in thorough learning and shows much better results compared to the old methods. This reduces the likelihood of human error and leads to more reliable results [57][58].
Recent automatic learning transition methodology (ML) has led to the conversion of brain tumor analysis using magnetic resonance imaging (MRI) to concentration learning (DL). The precision of results has been greatly enhanced, and the manual work needed for diagnosis has been reduced because of these models [48]. This progress is attributed to DL's ability to automatically pull-out traits from unprocessed data without needing manual steps, which has improved the quality of diagnoses and sped up medical choices [59].
Despite these advantages, ML and DL still encounter difficulties, such as the necessity for large, correctly labeled datasets for model training, as well as issues with interpretability, given that deep networks are frequently called a "black box" because understanding their decision-making processes is challenging [60]. To address these challenges and attain more dependable performance, assistance technologies including explainable AI tools, data augmentation strategies, and transfer learning may be used (ScienceDirect, 2024). Consequently, DL technologies are anticipated to be the future of brain tumor analysis since they have a great deal of promise for enhancing diagnosis and offering individualized treatment plans for every patient, which could lead to a considerable improvement in clinical results [59].
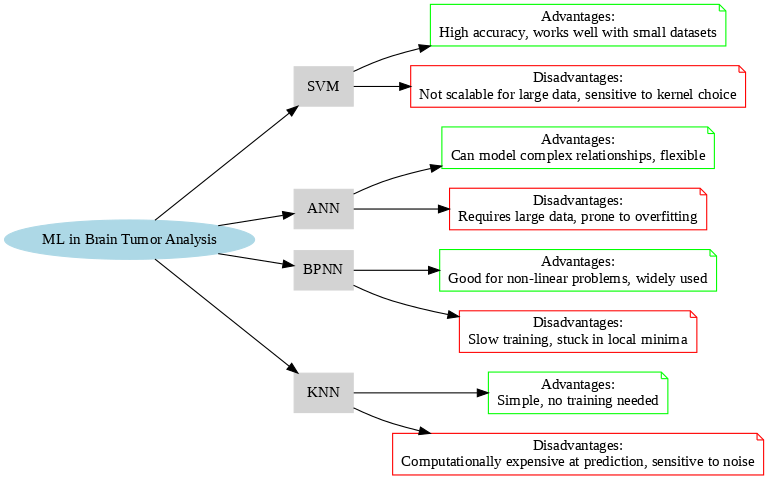
Figure 3. Comparison of ML Algorithms for Brain Tumor Analysis
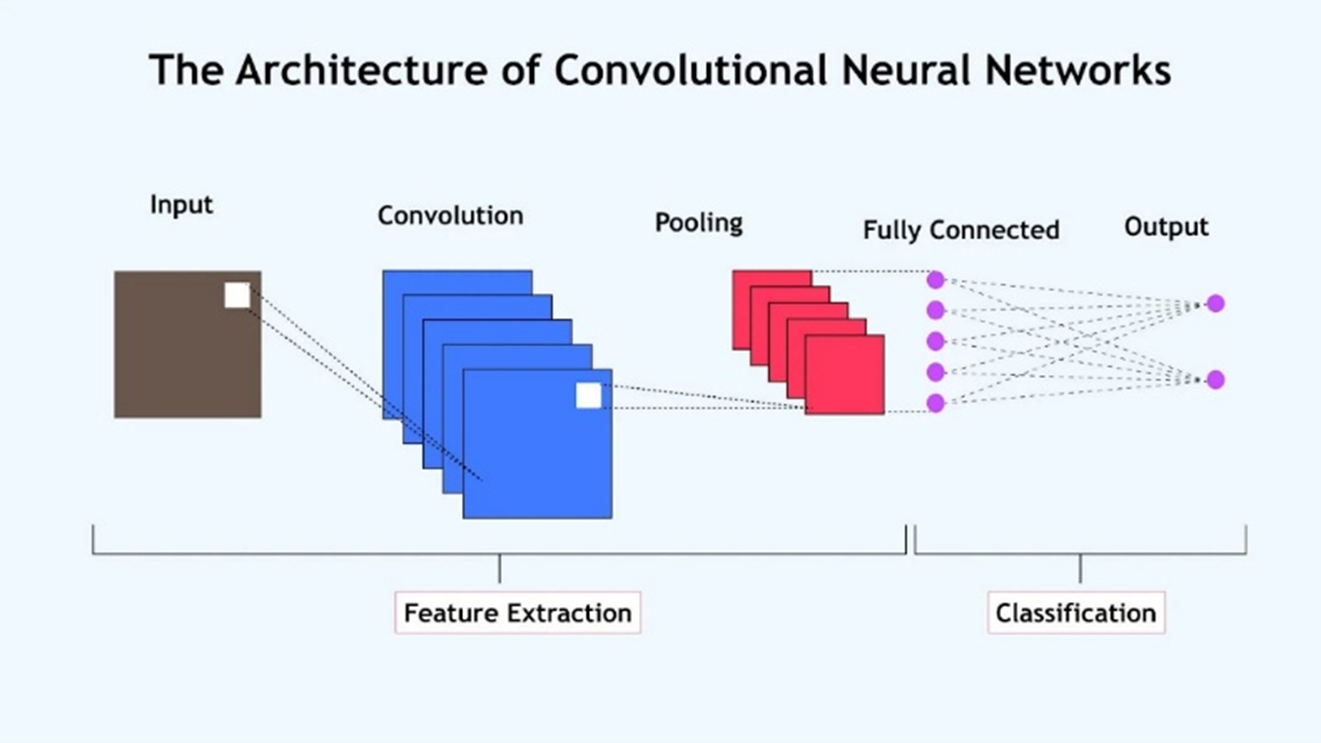
Figure 4. The CNN architecture, [56]
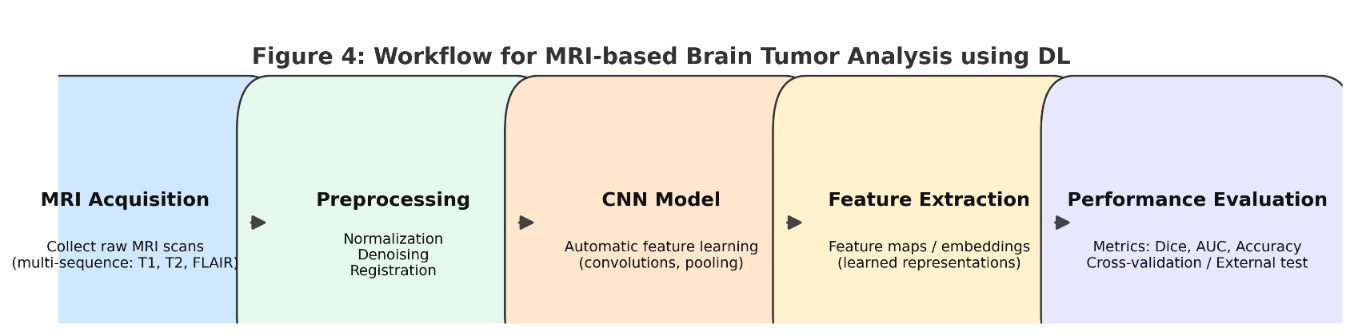
Figure 5. Workflow for brain tumor analysis using deep learning on MRI data
- PERFORMANCE ASSSESSMENTS
Performance metrics, which are used to gauge the effectiveness and precision of models, are a crucial component of brain tumor investigation employing AI techniques. The two most notable of these metrics are specificity, which evaluates the model's capacity to accurately identify benign (non-infected) cases, and accuracy, which displays the proportion of accurate predictions out of all cases. Other metrics are also employed, including Precision, which indicates the percentage of cases correctly classified as tumors out of all cases predicted by the model to be tumors; Loss, which quantifies the difference between predicted and actual values; Recall, which shows the model's capacity to detect all positive cases (tumors); and F1-score, which combines precision and recall into a single balanced metric [61].
- Accuracy (ACC)
Shows the proportion of samples that were accurately identified as tumors relative to the total number of samples. Its formula in mathematics is:
| 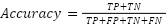
| (1) |
Where, 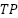 is the True positives (the tumor is present and detected).
is the True positives (the tumor is present and detected). 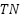 is the True negatives (there is no tumor and it is correctly classified).
is the True negatives (there is no tumor and it is correctly classified). 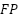 is the False positives (the model says there is a tumor when there is none).
is the False positives (the model says there is a tumor when there is none). 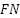 is the False negatives (the model says there is no tumor when there is none).
is the False negatives (the model says there is no tumor when there is none).
- Precision (P)
Calculates the percentage of cases out of all cases identified as tumors that were really correctly predicted to be tumors. The equation is:
| 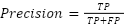
| (2) |
- Recall (R)
Shows that all true positives can be detected by the model. The formula for it is:
| 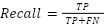
| (3) |
- Specificity (Sp)
This determines the proportion of actual negative events that the algorithm correctly categorizes as negative.
| 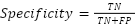
| (4) |
- F1-measure
This is computed by taking the accuracy and recall harmonic means. The highest level of accuracy and recall, or the ideal outcome, is indicated by a score of 1.
| 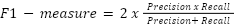
| (5) |
- MECHINE LEARNING FOR BRAIN TUMOR DETECTION AND CLASSIFICATIONS
This section shows and analyzes the different machine learning techniques for brain tumor detection and classifications. Figure 6 shows the stages of MRI-based brain tumor analysis. There are four primary types of brain tumor analysis tasks: detection, which involves identifying whether a tumor is present; segmentation, which involves identifying the tumor's exact location and size within MRI images; classification, which involves identifying the type and grade of the tumor; and, lastly, prediction of treatment prognosis or survival outcomes. Researchers have been used to achieve these objectives, particularly using a variety of artificial intelligence methods, as hybrid models that combine automated learning (ML), deep training (DL), and two approaches.
The basis of these systems is magnetic resonance imaging (MRI), which provides the highest level of accuracy and accuracy of brain visualization compared to other visualization methods. Using artificial intelligence techniques in the analytical process speeds up work, makes results more accurate, and provides early detection, accurate identification of tumors, and classification. It also helps to more reliably predict treatment outcomes. Therefore, as the researchers explain, the various methods used in these tasks will be considered in the following sections. We will look into how machine learning and deep learning can improve the accuracy and effectiveness of analyzing brain tumors.
Machine learning methods have become important in many areas of brain research using MRI, like telling different types of tumors apart, finding abnormal brain structures, detecting tumors, and predicting how long someone might live or how well treatments will work. These methods are valuable because they can mechanically detect worthwhile models and attributes from complicated brain imaging information, making them appropriate for managing the distinct hurdles tied to medical imaging and the structure of the brain. Consequently, machine learning strategies play a role in increasing the speed and correctness of evaluations by lowering the requirement for manual participation, which raises how well diagnoses are made and treatment plans are developed.
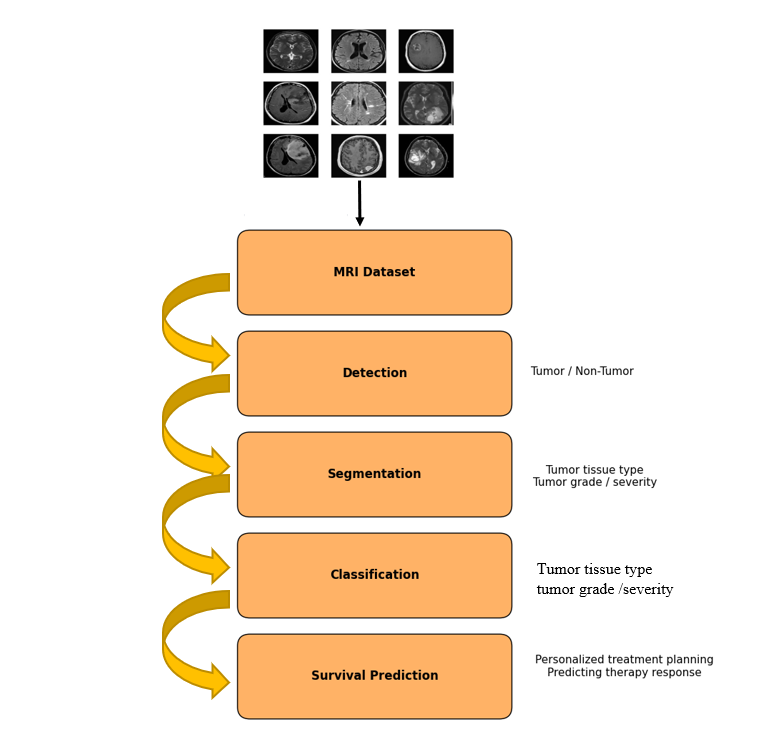
Figure 6. Stages of MRI-based brain tumor analysis
- Machine Learning-Based Tumor Detection
In their recent work, Khan M and colleagues (2022) [62] created a model using a deep neural network to identify brain tumors. The model was able to tell the difference between tumors and non-tumor cases very well, achieving an accuracy of 98% on a test set, with both precision and recall being very high. Another study shows that a mixed approach using functions such as density, texture, form, etc. of MRI images, as well as several SVM models formed on these functions, has resulted in extremely accurate over 97%. Dalal S et al. (2023) [63] used the hybrid method in related studies. First of all, they divided the data into groups using clustering methods such as K-mean, and then classified these groups using artificial neural networks (ANNs). The results of this method were similar to other studies on tumor cell segmentation, showing high accuracy and specificity when applying MRI data.
In recent years, many new methods have been developed to improve the accuracy of the diagnosis of brain tumors from MRI for example, Khan and his team (2022) [57] used a model based on fuzzy neural networks (ANFI) adapted to different situations after improving the images using advanced methods. This model has been successful in identifying and appropriate classifying information according to several datasets with success levels of 92% to 95%, helping to clearly distinguish between normal tissues and cancer zones. Bhimavarapu and his team (2024) [64] improved ANFI using genetic algorithms in a recent study, improving the accuracy of tumor classification compared to traditional methods. Meanwhile, wang and his team (2024) [51] used for MRI using a naive Bayesian algorithm to find tumors containing difficult parts like the central brain. Their method reached an accuracy of almost 91%, and it also made less errors in the distinction between cancer and healthy tissues.
In this regard, Stadlbauer and others (2022) [65] identified the benefits of integration of automated learning approaches, such as adaptive growth using random forests and radio strategies. The systems tested in over 100 models using patient data containing different types of tumors are achieved better than certain physicians with accuracy of >87%, unlike tumors. These results emphasize the potential for combining multiple image characteristics (such as intensity, shape, and physiological aspects) with complex algorithms to significantly improve early and accurate diagnoses. Additionally, they imply that increasing reliance on deep learning (DL) might streamline the initial data preparation steps and improve the results achieved later on.
Bhimavarapu and his team [64] presented a completely new way of finding and cutting out brain tumors in 2024. This new method uses how things look on the outside, along with the Fuzzy C-Means (FCM) algorithm, to split up magnetic resonance imaging (MRI) scans by things like how rough or smooth they are and how strong the colors are. Once they had separated the tumors, the scientists used the Extreme Learning Machine (ELM) algorithm to sort them with very high correctness. When they tested this method on different groups of information, such as Figshare, SARTAJ, and Br35H, the results were really positive. It worked better than older methods by between 1.21% and 6.23%. It achieved a correctness level of 98.56%, a prediction accuracy of 99.14%, and a recall rate of 99.25%. These results show that this new method could be used in hospitals and clinics because it makes diagnoses faster and reduces th chance of mistakes by doctors.
Zhou and others (2023) [44] showed that combining deep clustering with deep learning methods in mixed frameworks can greatly improve the accuracy of segmentation. This potential for using these methods in real-world medical settings is supported by Deepa S and colleagues (2022) [66], who proved that mixing ELM techniques with MRI images can significantly improve the accuracy of predictions. Table 1 and Figure 7 show and analyze some machine-learning-based approaches for brain tumor detection.
Table 1. Accuracy of Different ML Methods for Brain Tumor Analysis
Reference | Method | Dataset | Accuracy |
[67] | Feed-forward & Back-propagation Neural Network | 239 MRI images | 99% |
[68] | K-means Clustering | BraTS 2015 | 94.07% |
[69] | K-means Clustering + SVM | Harvard, RIDER, and Local datasets | 97.1% |
[70] | NSCT + ANFIS | BraTS 2015 Leaderboard and Challenge | 95.9% / 96.4% |
[71] | Naïve Bayes Classification | 50 MRI images | 94% |
[72] | Adaptive Boosting + Random Forest | Radiomic features from 167 patients | 87.5% |
[64] | Fuzzy C-means + Extreme Learning Machine | Figshare, SARTAJ, Br35H datasets (combined) | 98.56% |
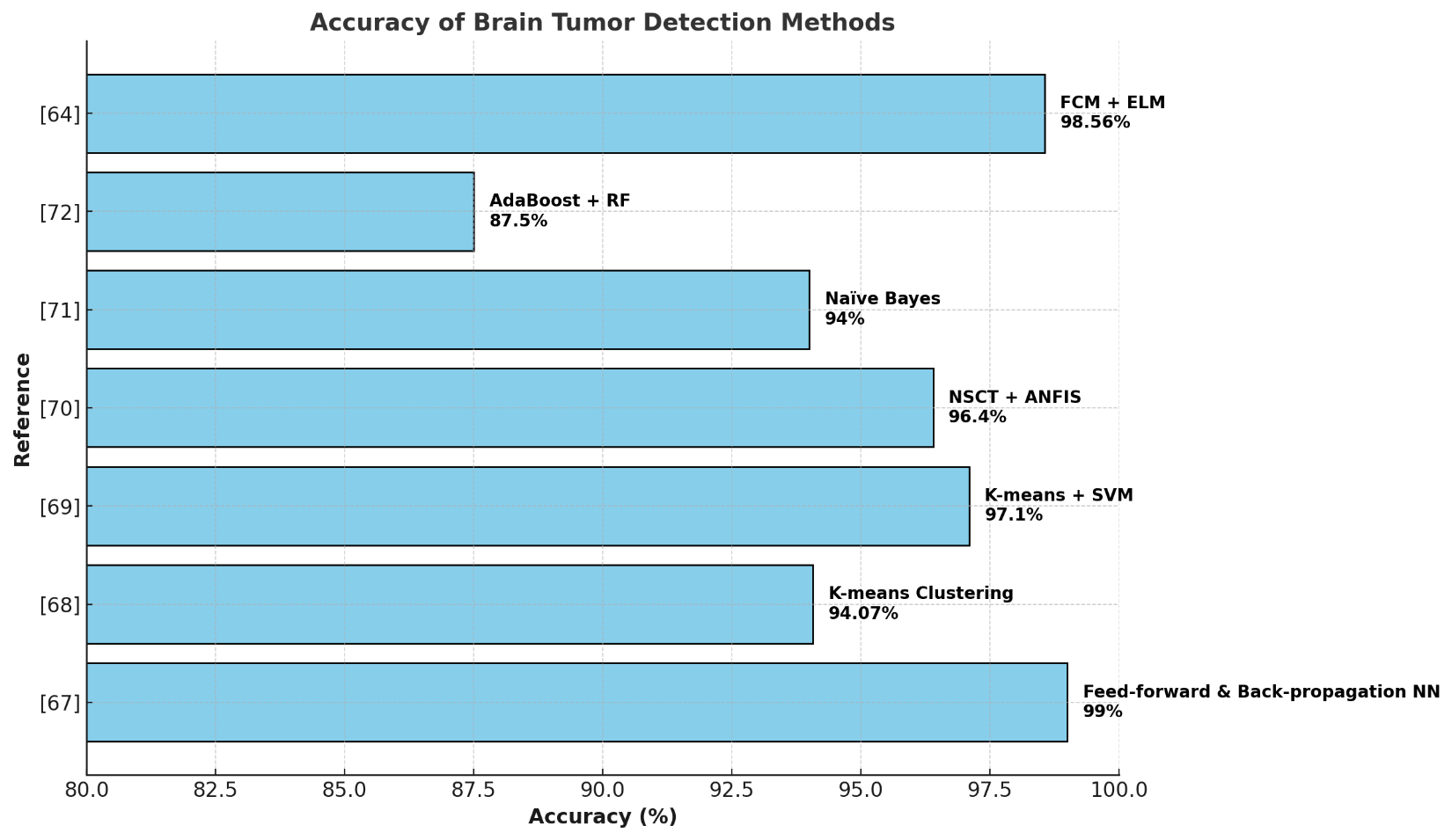
Figure 7. Accuracy of Different ML Methods for Brain Tumor Analysis
- Machine Learning-Based Tumour Classification
Çınarer and colleagues, in their 2023 study [73], revealed that several machine learning methods, including support vector machines (SVM), the k-nearest neighbor algorithm (KNN), random forest (RF), and linear discriminant analysis (LDA), have the potential to classify different kinds of brain tumors like gliomatosis, multi-focal, and multicentric. These classifications are based on statistical characteristics obtained from magnetic resonance imaging (MRI) scans. The research emphasized the ability of SVM to effectively manage complex medical data, as demonstrated by its 90% accuracy in classifying tumors. Furthermore, Ramdlon and associates (2022) [74] presented a new strategy utilizing the KNN algorithm to improve the accuracy of identifying brain tumors and guiding treatment approaches. This technique extracts tumor sections by employing morphology and watershed algorithms, after first enhancing images and converting them to binary format during a preprocessing stage. Subsequently, features from brain CT images (T1 and T2) are analyzed to determine the type of tumor, such as astrocytoma, glioblastoma, and oligodendroglioma. The system reaches 89.5% accuracy and shows how it can help physicians make the best treatment decision.
The MRI classification intellectual system developed by Margalani et al. [75]. can help identify mild conditions, Alzheimer's disease, tumors, and other brain disorders. This system was very effective in using function method methods and distinguishing these conditions, reaching almost 97% of accuracy. This makes it a useful tool for accelerating diagnosis and reducing reliance on traditional human analysis. After that, Rao et al. [76] introduced a more complete model that improved contrast and improved image quality using normalized median filters (NMF), followed by binomial thresholds for individual tumors. They also used GLCM and SGLDM to extract important features.
The classification process, which separates benign and malignant tumors and evaluates the severity of malignant ones, was handled using KSVM and SSD algorithms. Additionally, the Harris Hawks Optimization (HHO) method was used to improve feature selection. When tested on BraTS data (2018–2020), this method showed promising results with accuracy levels of 99.2%, 99.36%, and 99.15%, indicating strong potential for helping in medical diagnosis and treatment plans. These results align with recent studies, including those by Rao et al. and Mandle et al. [76][77] which have shown how AI methods can improve the accuracy and speed of medical diagnoses using MRI images. Table 2 and Figure 8 show and analyze some of the machine-learning-related works for brain tumor classification.
Table 2. Machine-learning-related works for brain tumor classification
Reference | Method | Dataset | Accuracy |
[73] | KNN, RF, SVM, and LDA | REMBRANDT | 90% (for SVM) |
[74] | k-nearest neighbor (KNN) | TCIA | 89.5% |
[75] | Bag of Features module | TCIA, XNAT, and Oasis | 97% |
[76] | KSVM and SSD classifiers | BRATS 2018, 2019, and 2020 | 99.2%, 99.36%, 99.15% |
[77] | Kernel-based SVM (K-SVM) | 160 MRI images | 98.75% |
[78] | SVM with RBF and linear kernels | Dataset derived from internet | 87.5% |
[79] | Five ML models | 1000 MRI images | 98.51% (for KNN) |
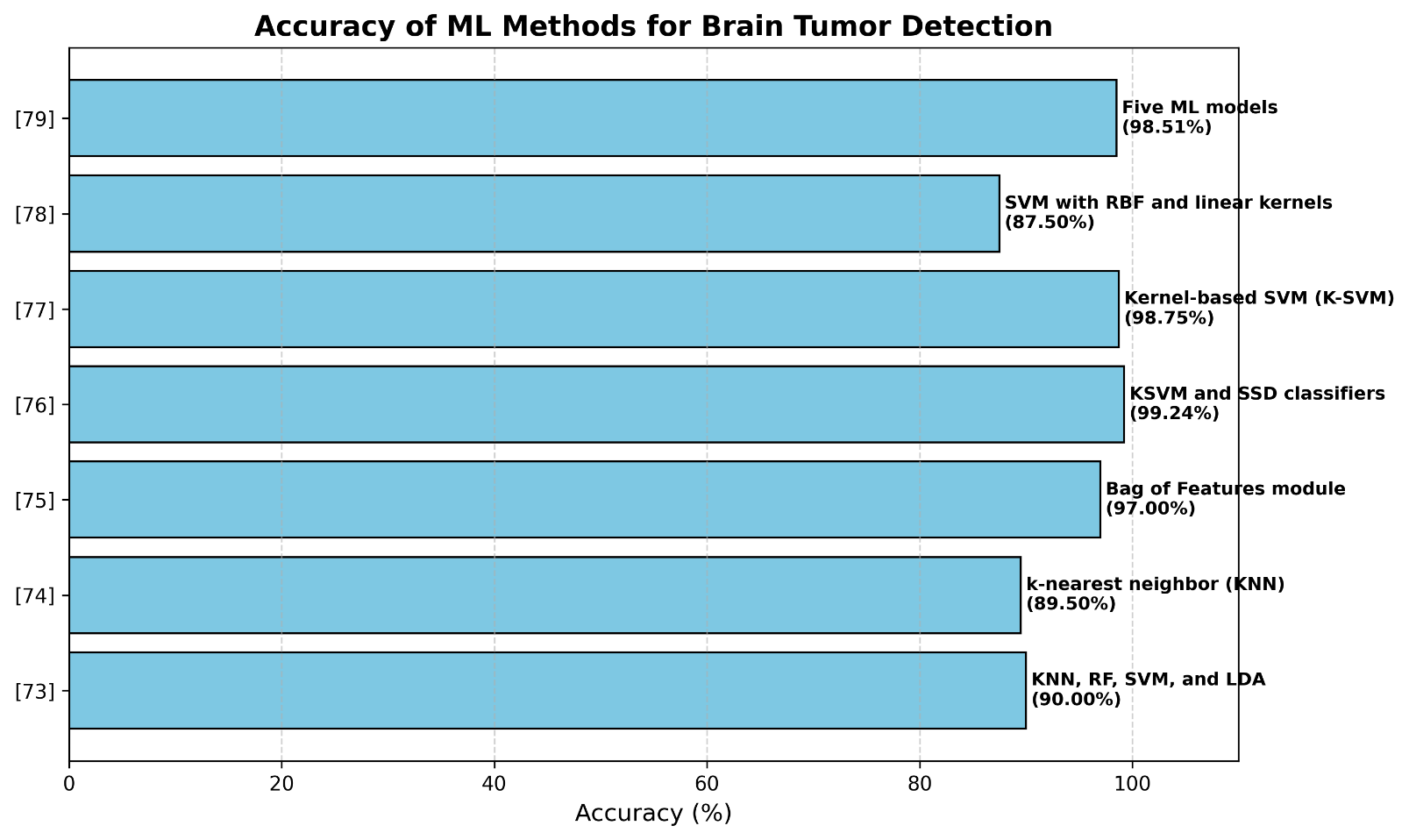
Figure 8. Machine-learning-related works for brain tumor classification
- DEEP LEARNING FOR BRAIN TUMOR DETECTION AND CLASSIFICATIONS
The deep training methods used in MRI analysis have become one of the most sophisticated non-invasive tools to correct the importance of tasks such as tumour search, understanding and organizing abnormal brain structures, and predicting survival potential. Unlike older or standard automated learning approaches, deep learning models can automatically find complex, layered models in MRI data without manually selecting functions or performing the first stage of detection. This is especially important for brain visualization. Complex and changing brain structures are extremely important for a suitable diagnosis, meaning that even small models can be predicted to determine how the condition progresses. Deep training is a robust tool that helps make the medical assessment of the brain more accurate and reliable, which improves patient diagnosis and treatment outcomes. New research shows that deep learning can combine detailed high-level information with basic features found in MRI data.
- Deep Learning-Based Tumor Detection
In 2023, a reliable way to find brain tumors using MRI scans was created by Musallam and their team [80]. They used a deep convolutional neural network (DCNN) that had a special three-step setup and unique design. The network used bigger filters that were 7×7×7 in size, included batch normalization, and had a simple structure with fewer layers. This helped the system correctly sort images into four groups: normal, pituitary tumors, meningiomas, and gliomas. When tested on 3,394 MRI images, the method was accurate 98.22% of the time, making it easier for doctors to diagnose and treat patients more effectively, which helps improve patient outcomes.
As deep learning methods for MRI analysis quickly improved, Nayak and their team (2023) [81] introduced a new model called Dense EfficientNet. This model was able to classify T1-weighted contrast-enhanced MRI images from 3,260 samples into four categories: meningioma, glioma, pituitary tumor, and no tumor. The model performed very well, with an F1-score of 98% and high accuracy of 99.97% on training data, and 98.78% on test data, showing its potential for early diagnosis and helping doctors make better decisions.
In 2022, Obeidavi and their team [82] built an automated system using a residual convolutional neural network (Residual CNN) with 11 skip-connections. This allowed the model to learn features from different levels and handle the vanishing gradient problem. Using the BraTS 2015 dataset, the system was effective at processing complex images, achieving 94.43% accuracy, an average IoU of 54.21%, and a Weighted IoU of 93.64%. Mahjoubi and their team (2023) [83] developed a model based on an improved standard CNN, which included pooling and dense layers and five convolutional layers with a total of 32 to 512 filters. This model was tested on a combined dataset from Figshare, SARTAJ, and Br35H, and showed good results with an accuracy of 95.44%, a recall of 95%, and an F1-score of 95.36%, using ReLU and SoftMax activation functions. This not only supports early detection and treatment but also enhances accuracy in identifying brain tumors.
Hashan et al. [84] presented a method based on a convolutional neural network (CNN) optimized through the Adam algorithm for analyzing brain MRI images for tumor recognition. Their model surpassed previous techniques such as SVM and MFDFA + Random Forest, which had lower accuracies of 81.47% and 86.7%, respectively, achieving an accuracy of 90% and an F-score of 89% when evaluated on a dataset of 400 images. Another investigation by Bhanothu et al. [85] proposed a Faster R-CNN-based approach for identifying tumors in MRI images and classifying them as pituitary, meningioma, or glioma, utilizing a VGG-16 network. When tested on a dataset of 233 patients, this technique, which used bounding boxes for identification and classification of tumors, achieved accuracy rates of 75.18% for gliomas, 89.45% for meningiomas, and 68.18% for pituitary tumors, resulting in an overall mean accuracy (mAP) of 77.60%.
A new and simpler version of the U-Net model, named LeU-Net, was introduced by Rai et al. [86]. It was made specifically for quickly and accurately finding brain tumors in MRI scans. This model mixes the best parts of LeNet and U-Net to create a lighter and faster structure. It improves accuracy and makes processing faster, especially when there's not a lot of data. When tested on 253 MRI scans, both cropped and not cropped, the system had an accuracy of 98% for the cropped images and 94% for the uncropped ones. In another study, Mohan et al. [87] developed a U-Net based CNN method for detecting and classifying brain tumors. They focused on early diagnosis to help stop serious health issues from getting worse and used MRI’s special features to find tumors more precisely. Their tests on 3,264 MRI images showed the model works well, achieving 98.67% accuracy, 96.72% sensitivity, and 94.86% specificity. These results show the model could be useful in hospitals for early detection of brain tumors and for helping plan effective treatments.
Aamir et al. [26] created an automatic way to detect brain tumors using MRI. Their process starts with making medical images clearer, then uses two already trained deep learning models to find important parts of the images. They used Partial Least Squares to combine these features into a single set of features, and Agglomerative Clustering to spot possible tumor areas. They identified how well the method worked with data from 233 patients and found the accuracy of the classification of 98.95%.
Almadhoun et al. [88] used deep training to point out the differences between primary and secondary tumors in the brain on MRI. They tested several detailed training models, including the first meanings, VGG16, ResNet50, MobileNet, and their own models. The results show that these models are highly effective using their own models, reaching the accuracy of the F1 98.28% indicator, starting at 99.88%, VGG16 at 99.86% and ResNet50 at 98.14%. MobileNet had a lower accuracy of 88.98% during testing with a larger set of 10,000 MRIs of the brain, but these results show that the accuracy of brain tumor research and classification can be increased, indicating that physicians can help doctors at work. Table 3 and Figure 9 show and analyze some of deep-learning-related works for brain tumor detection.
Table 3. Deep-learning-related works for brain tumor detection
Reference | Method | Dataset | Accuracy |
[80] | Deep CNN (DCNN) | 3,394 MRI images | 98.22% |
[89] | CNN | 3,264 MR images | 93.3% |
[81] | Dense EfficientNet | 3,260 T1-weighted MRI images | 98.78% |
[82] | Residual CNN | BraTS 2015 | 94.43% |
[83] | CNN | Figshare, SARTAJ, Br35H datasets | 95.44% |
[84] | Optimized CNN | 400-image dataset | 90% |
[85] | Faster R-CNN | 233 patients’ MRI dataset | 77.60% |
[86] | LeU-Net | 253 MRI images | 98% and 94% |
[87] | U-Net CNN | 3,264 MRI images | 98.67% |
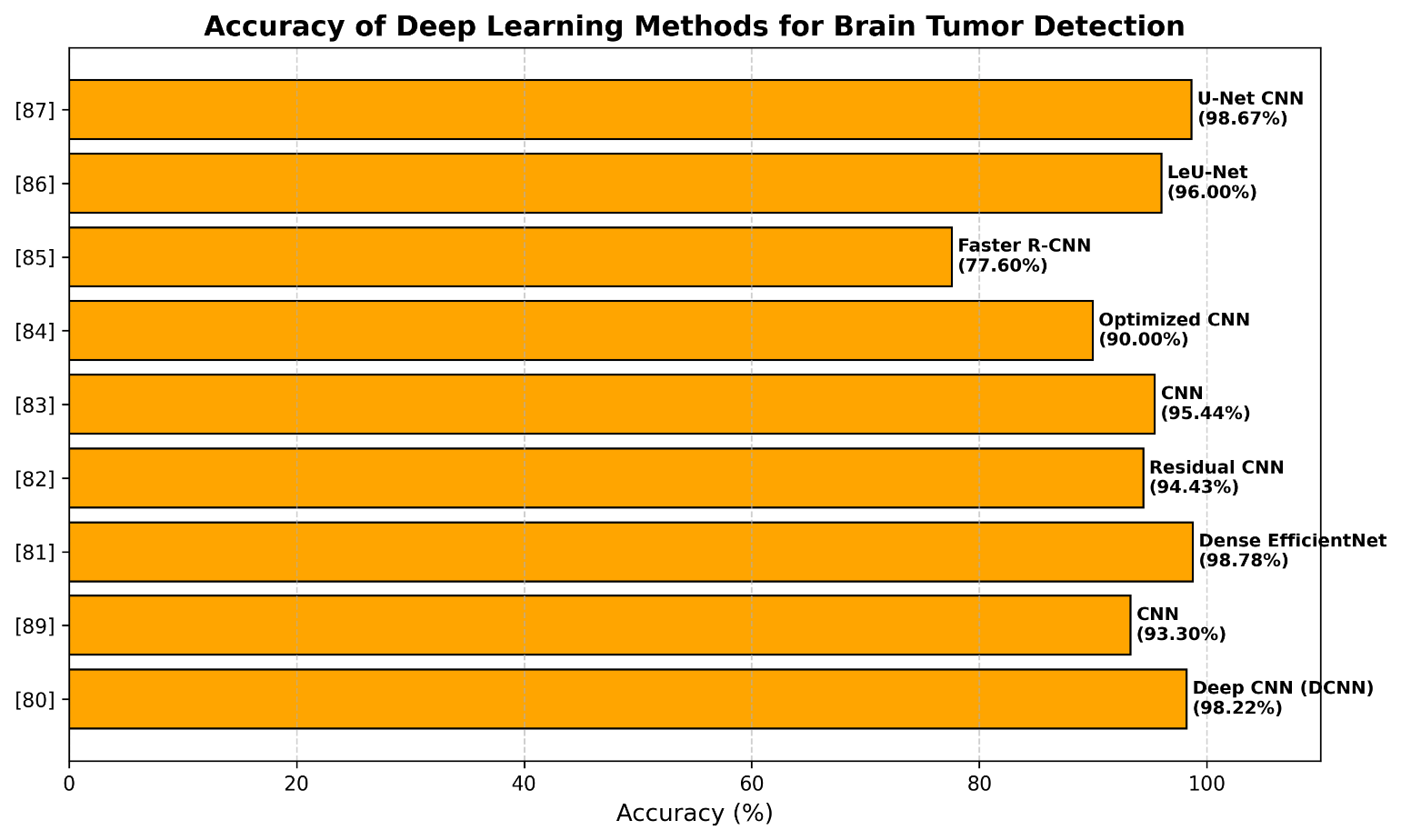
Figure 9. Deep-learning-related works for brain tumor detection
- Deep Learning-Based Tumour Classification
A new simpler version of the U-Net model called Leu-Net was developed by Rai et al. [86] Detect the brain tumors quickly and with precision during the MRI scanner. This model combines the best parts of LENET and U-NET, which contributes to increased accuracy and speed, especially when using small data. When tested on 253 MRI scans, both with and without parts of the image removed, the system showed 98% accuracy for the cropped images and 94% for the full images.
In another study, Mohan et al. [87] created a U-Net-based CNN method for finding and classifying brain tumors. Their approach aimed to help with early detection to prevent serious health issues and used MRI features to precisely locate tumors. The model performed very well in tests with 3,264 MRI images, reaching 98.67% accuracy, along with 96.72% sensitivity and 94.86% specificity. These results show that this model can help detect early tumor detection and better treatment plans in real hospitals.
Deepak et al. [31]created an appropriate model for classifying brain tumors using critical learning communication. First of all, they taught GoogleNet models on the ImageEnet dataset, then constituted the final part of the network and used a set of FigShare data to classify tumors in three groups. They used deep network functions with different classification methods such as KNN, SVM, SoftMax, and more. After using a five-stroke cross-check, the results showed that this method works better than many other approaches, reaching an average accuracy of 98%.
Kumar et al. [90] developed a complete method for diagnosing brain tumors using MRI images using publicly accessible data. Those techniques used CNNs in transmission training with preformed models such as ResNet-50, VGG-16, and U-NET to improve image segmentation. Next, I improved the model using transformations. This model reached 93.56% accuracy and 92.19% reviews of benign tumors, with 92.45% accuracy and 92.45% reviews of high-performance malignant tumors. The dataset included 1,572 IMR IMM images T1W, with F1 indicators of 92.33% and 93.92% of benign and malignant tumors, respectively. These results show to the extent that a relative learning helps physicians make better decisions and improve brain tumor classification.
A lightweight U-Net model called LeU-Net was created by Rai et al. [86]. It's meant to find tumors in brain MRIs quickly and accurately. This model uses parts of both LeNet and U-Net to make it more efficient and precise, especially when working with smaller sets of data. When tested on 253 MRI scans, both cropped and not cropped, the system got 98% accuracy for the cropped images and 94% for the uncropped ones. In another study, Mohan et al. [87] developed a U-Net CNN method for finding and categorizing brain tumors. Their approach focused on early diagnosis to stop serious health issues from getting worse. They used MRI features to locate tumors accurately. Their tests on 3,264 MRI images showed very good results with 98.67% accuracy, 96.72% sensitivity, and 94.86% specificity. These results show the model could be useful in clinics for early detection and better treatment planning.
Asif et al. [91] used five modern deep learning models—InceptionResNetV2, DenseNet121, ResNet152V2, Xception, and DenseNet201. Each of these models has a deep dense block and a SoftMax output layer. They used these models to build a more advanced deep learning and transfer learning method for classifying brain tumors. After refining on the Figshare dataset, the Xception architecture showed its high efficiency in helping physicians make accurate and timely diagnostic decisions, with an accuracy rate of 99.67% for a three-class classification and 95.87% for a four-class classification. Meanwhile, Haq and others [92] suggested using two separate structures that rely on convolutional neural networks (CNNs). Their first network, evaluated on the BraTS2018 dataset, classified tumors into glioma, meningioma, and pituitary types with an accuracy of 97.3% and a Dice similarity coefficient (DSC) of 95.8%, while the second network recorded an accuracy of 96.5% and a DSC of 94.3%.
In another study, Rasheed et al. [93] created a special technique that uses a deep CNN with several layers. These layers include convolution, batch normalization, dropout, max pooling, global average pooling, and dense layers that use L1 and L2 regularization. When evaluated on 3064 MRI images, the model achieved a classification accuracy of 98.04% by using the SoftMax activation function and the Adam optimizer with ReduceLROnPlateau during training. Kumar and his team [90] created a 25-layer neural network called CNNs, which helped to more accurately classify brain tumors using MRI. They used Star IRM images so that the model could find models and details in different layers. Using the Adam method, the model received an accuracy of 86.23% and reached 81.6% using the Sadam method.
In another study, Joshi and his group [94] used a set of Brats 2020 data to create a new learning method to classify and isolate brain tumors. The model using ResNet-50 has an accuracy of 97.8%, and 96.9% for ResNet-101. For the model to work better with different types of data, methods such as rotating and rotating images are used, as well as transmission training. To find tumors more accurately, they added jump connections and dumping layers to the U-NET model. With these changes, the model helped to obtain 98.5% bone similarity (DSC). This indicates that it is useful in real-world medical situations, is more accurate and faster than previous models.
Haque and his team [95] created a new model called the Neuronet 19 based on the structure of the VGG19. They added an inverted pyramid (IPPM) to help the model find features of different sizes. When testing the MRI 7023 MRI scan, he showed excellent results with an accuracy of 99.3%. This suggests that this is a valuable tool for physicians for early detection and classification of brain tumors, and can improve patient diagnosis and care.
However, in order to improve classification accuracy, Mohanty et al. [96] suggested a novel method based on Convolutional Neural Networks (CNNs) aided by a Soft Attention mechanism. characteristics are retrieved from the four layers of the suggested model to create a comprehensive feature vector. An attention mechanism that concentrates on the most significant characteristics is then used to improve the choice. With a specificity of 87.41% utilizing Figshare data, the model showed good performance in diagnosing pituitary tumors, meningioma, and glioma, with high accuracy (95.57%, 94.61%, 95.16%), recall (93.64%, 96.88%, 94.65%), and F1-scores (94.45%, 95.98%, 95.00%) for each type, respectively.
Using BRATS data, Jain et al. [97] introduced an inventive ensemble approach (Ensemble Deep Learning – EDL-BTC) for the early detection of brain tumors. In order to extract features from tumor photos, this method combines many pre-trained models, including ResNet50, InceptionV3, and MobileNetV2. ReLU and Dense layers are then used as a classification tool. The model outperformed numerous other state-of-the-art models, demonstrating great efficiency across cross-validation tests (5-, 10-, and 20-fold), with an accuracy range from 98.3% to 98.6%. These findings validate how deep ensemble models help physicians speed up diagnosis and enhance tumor classification accuracy, both of which have a direct impact on better patient care and clinical results.
Hanan Omran et al. [98] presented two models were trained and tested using a publicly accessible brain MRI dataset from Kaggle, which included 7023 contrast-enhanced pictures classified into four classes: glioma, meningioma, pituitary tumour, and no tumour. To ensure a fair assessment, the data was divided into three subgroups: testing, validation, and training. The VGG19-SVM (RBF) model achieved 96.2% validation and 97.8% testing accuracy, whereas the VGG19-Softmax model obtained 99% training and 98.4% validation accuracy. Table 4 and Figure 10 show and anlyze some of the deep-learning-related works for brain tumor classification.
Table 4. Deep-learning-related works for brain tumor classification
Reference | Method | Dataset | Accuracy |
[31] | Deep transfer learning model | Figshare | 98% |
[99] | CNN and transfer learning | 1572 T1w MRI images | - |
[91] | Deep transfer learning | Figshare | 99.67% / 95.87% |
[92] | CNNs | BraTS 2018 | 97.3% / 96.5% |
[93] | ConvNet | 3064 MRI images | 98.04% |
[90] | 25-layer CNN | 3064 MRI images | 86.23% / 81.6% |
[94] | DL (ResNet and U-Net) | BRATS 2020 | 97.8% / 96.9% |
[95] | NeuroNet19 | 7023 MRI images | 99.3% |
[96] | CNN and a soft attention mechanism | Figshare | - |
[97] | Ensemble deep learning and transfer learning | BraTS | 98.3% / 98.6% / 98.6% |
[100] | Attention-Gated Recurrent Units (A-GRU) | BTD dataset | 99.32% |
[101] | Deep CNN | 7000 MRI images | 94–97% |
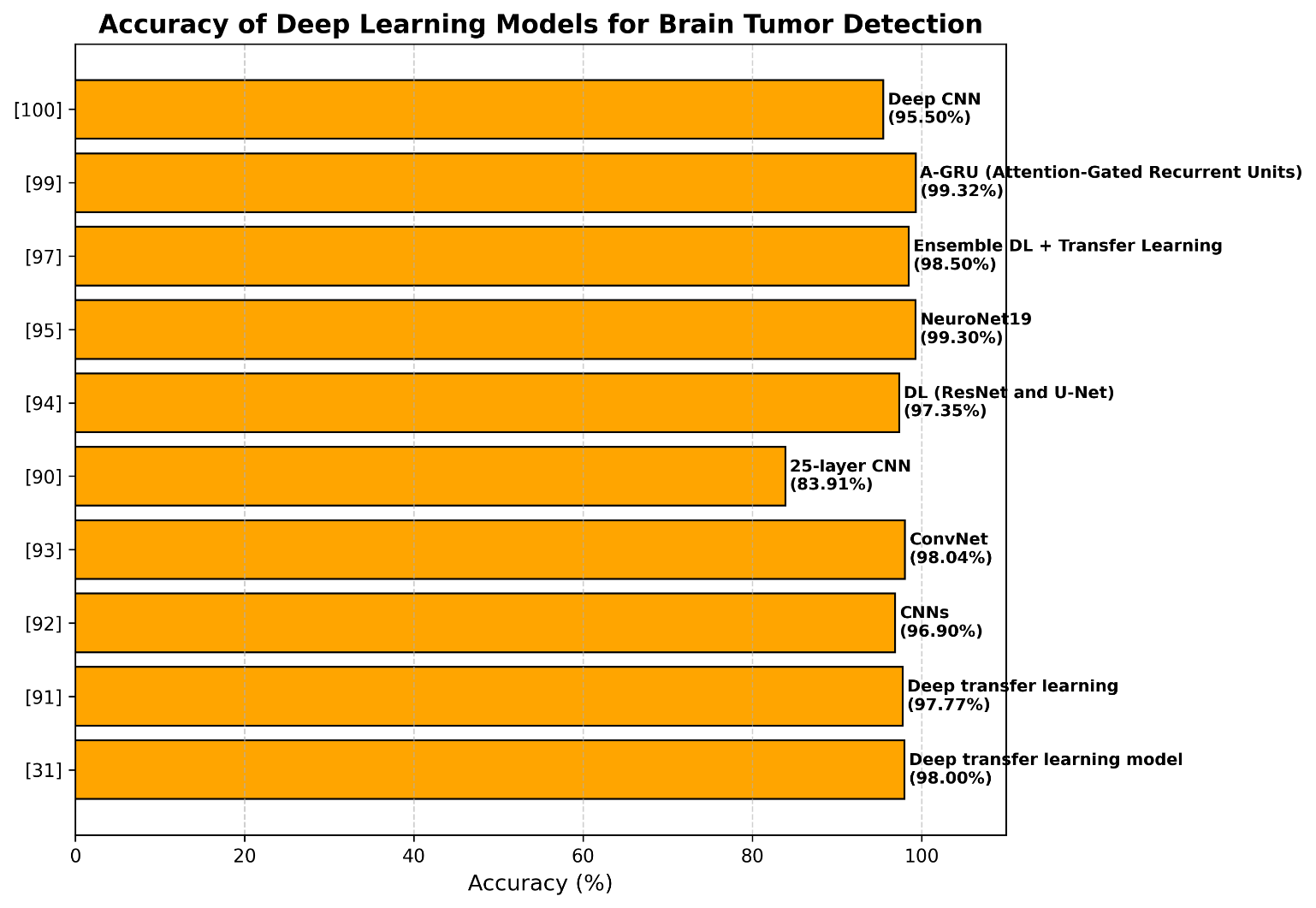
Figure 10. Deep-learning-related works for brain tumor classification
- DISCUSSION AND RESEARCH TRENDS
The evaluated and discussed studies show a number of recurring patterns in the analysis of brain tumours using MRI. Convolutional neural network (CNN) architectures, in particular, are the most popular deep learning models in recent research and routinely outperform conventional machine learning techniques. Transfer learning using pretrained networks like VGG, ResNet, DenseNet, and EfficientNet has become a popular tactic, particularly when working with a little amount of annotated medical data. Furthermore, by integrating complimentary feature representations, ensemble and hybrid models often perform better than single-model architectures.
Deep learning models' improved performance can be explained by their capacity to automatically extract discriminative and hierarchical features from unprocessed MRI data. CNN-based models are better at capturing intricate spatial patterns and tumour heterogeneity than classic machine learning techniques, which mostly depend on manually created features and domain knowledge. This capacity is especially crucial for addressing differences across MRI sequences and differentiating visually similar tumour kinds. Even though a number of studies have claimed high accuracy rates, particularly those that surpass 98–99%, care must be used when interpreting these findings. Overfitting was more likely because many models were tested on small or homogeneous datasets. Additionally, performance differs greatly amongst datasets like BraTS, Figshare, and private clinical collections, suggesting difficulties with model generalization and practical clinical application.
All of the examined researches still have a number of unresolved issues. These include a lack of standardized evaluation processes, a lack of well-annotated datasets, a lack of model interpretability, and data heterogeneity resulting from differences in MRI acquisition protocols. In order to convert research models into trustworthy clinical decision support systems, these issues must be resolved.
- CONCLUSION
This review methodically looked at new deep learning and machine learning techniques for MRI-based brain tumour classification, segmentation, and detection. The results of the examination of 67 studies released between 2019 and 2024 demonstrate unequivocally that deep learning models—in particular, architectures based on convolutional neural networks—have emerged as the most popular and successful method for automated brain cancer analysis. These models' capacity to autonomously extract intricate and hierarchical information from medical images is partly responsible for their continuously higher accuracy and robustness when compared to conventional machine learning techniques. Despite these encouraging outcomes, there are still a few restrictions. Concerns about model generalization and potential overfitting are raised by the fact that many studies rely on small or homogeneous datasets. Moreover, difficulties associated with.
The broad use of these models in actual medical settings is still hampered by data heterogeneity among MRI acquisition techniques, a lack of model interpretability, and inadequate external clinical validation. Future work should concentrate on creating hybrid and ensemble models that combine the resilience and interpretability of conventional machine learning methods with the powerful representation learning capacity of deep learning. Furthermore, to increase model dependability and clinical application, large-scale, heterogeneous clinical datasets, standardized evaluation methodologies, and multi-modal imaging data must be integrated. Overall, this review emphasizes the substantial potential of AI-based techniques to improve brain tumour diagnosis and facilitate individualized treatment planning. Sustained cooperation among medical professionals and data scientists will be essential in converting these sophisticated algorithms from research environments into trustworthy clinical decision support systems.
DECLARATION
Supplementary Materials
All data is included in the paper.
Author Contribution
All authors contributed equally to this paper. All authors read and approved the final paper.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
REFERENCES
- D. L. Barabási et al., “Neuroscience needs network science,” Journal of Neuroscience, vol. 43, no. 34, pp. 5989–5995, 2023, https://doi.org/10.1523/JNEUROSCI.1014-23.2023.
- L. K. Fellows, “The cognitive neuroscience of human decision making: a review and conceptual framework,” Behav Cogn Neurosci Rev, vol. 3, no. 3, pp. 159–172, 2004, https://doi.org/10.1177/1534582304273251.
- I. Ilic and M. Ilic, “International patterns and trends in the brain cancer incidence and mortality: An observational study based on the global burden of disease,” Heliyon, vol. 9, no. 7, p. e18222, 2023, https://doi.org/10.1016/j.heliyon.2023.e18222.
- D. N. Louis et al., “The 2021 WHO classification of tumors of the central nervous system: a summary,” Neuro Oncol, vol. 23, no. 8, pp. 1231–1251, 2021, https://doi.org/10.1093/neuonc/noab106.
- D. Hanahan and R. A. Weinberg, “Hallmarks of cancer: the next generation,” Cell, vol. 144, no. 5, pp. 646–674, 2011, https://doi.org/10.1016/j.cell.2011.02.013.
- V. Labi and M. Erlacher, “How cell death shapes cancer,” Cell Death Dis, vol. 6, no. 3, pp. e1675–e1675, 2015, https://doi.org/10.1038/cddis.2015.20.
- R. He et al., “Mechanisms and cross-talk of regulated cell death and their epigenetic modifications in tumor progression,” Mol Cancer, vol. 23, no. 1, p. 267, 2024, https://doi.org/10.1186/s12943-024-02172-y.
- J. S. Brown, S. R. Amend, R. H. Austin, R. A. Gatenby, E. U. Hammarlund, and K. J. Pienta, “Updating the definition of cancer,” Molecular Cancer Research, vol. 21, no. 11, pp. 1142–1147, 2023, https://doi.org/10.1158/1541-7786.MCR-23-0411.
- Q. T. Ostrom et al., “CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2015–2019,” Neuro Oncol, vol. 24, no. Supplement_5, pp. v1–v95, 2022, https://doi.org/https://doi.org/10.1093/neuonc/noac202.
- M. A. Vogelbaum et al., “Treatment for brain metastases: ASCO-SNO-ASTRO guideline,” Oxford University Press US, 2022, https://doi.org/10.1093/neuonc/noab262.
- S. E. Mousavi, H. Seyedmirzaei, S. Shahrokhi Nejad, and S. A. Nejadghaderi, “Epidemiology and socioeconomic correlates of brain and central nervous system cancers in Asia in 2020 and their projection to 2040,” Sci Rep, vol. 14, no. 1, p. 21936, 2024, https://doi.org/10.1038/s41598-024-73277-z.
- J. Huang, H. Li, H. Yan, F.-X. Li, M. Tang, and D.-L. Lu, “The comparative burden of brain and central nervous system cancers from 1990 to 2019 between China and the United States and predicting the future burden,” Front Public Health, vol. 10, p. 1018836, 2022, https://doi.org/10.3389/fpubh.2022.1018836.
- Y. Fan et al., “Burden and trends of brain and central nervous system cancer from 1990 to 2019 at the global, regional, and country levels,” Archives of Public Health, vol. 80, no. 1, p. 209, 2022, https://doi.org/10.1186/s13690-022-00965-5.
- M. Weller et al., “European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas,” Lancet Oncol, vol. 18, no. 6, pp. e315–e329, 2017, https://doi.org/10.1016/S1470-2045(17)30194-8.
- F. Bertolini, A. Spallanzani, A. Fontana, R. Depenni, and G. Luppi, “Brain metastases: an overview,” CNS Oncol, vol. 4, no. 1, pp. 37–46, 2015, https://doi.org/10.2217/cns.14.51.
- F. Bray et al., “Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries,” CA Cancer J Clin, vol. 74, no. 3, pp. 229–263, 2024, https://doi.org/https://doi.org/10.3322/caac.21834.
- P. Y. Wen and R. J. Packer, “The 2021 WHO classification of tumors of the central nervous system: clinical implications,” Oxford University Press US, 2021, https://doi.org/https://doi.org/10.1093/neuonc/noab120.
- F. J. Dorfner, J. B. Patel, J. Kalpathy-Cramer, E. R. Gerstner, and C. P. Bridge, “A review of deep learning for brain tumor analysis in MRI,” NPJ Precis Oncol, vol. 9, no. 1, p. 2, 2025, https://doi.org/10.1038/s41698-024-00789-2.
- M. F. Ahamed et al., “A review on brain tumor segmentation based on deep learning methods with federated learning techniques,” Computerized Medical Imaging and Graphics, vol. 110, p. 102313, 2023, https://doi.org/10.1016/j.compmedimag.2023.102313.
- M. Lather and P. Singh, “A Comprehensive Review on Strategies to Detect, Diagnose and Classify Brain Tumors,” Biomedical and Pharmacology Journal, vol. 16, no. 4, pp. 1915–1926, 2023, https://doi.org/https://dx.doi.org/10.13005/bpj/2770.
- S. Bauer, R. Wiest, L.-P. Nolte, and M. Reyes, “A survey of MRI-based medical image analysis for brain tumor studies,” Phys Med Biol, vol. 58, no. 13, p. R97, 2013, https://doi.org/10.1088/0031-9155/58/13/R97.
- C. Catana, A. R. Guimaraes, and B. R. Rosen, “PET and MR imaging: the odd couple or a match made in heaven?,” Journal of Nuclear Medicine, vol. 54, no. 5, pp. 815–824, 2013, https://doi.org/10.2967/jnumed.112.112771.
- Q. Luo, Y. Li, L. Luo, and W. Diao, “Comparisons of the accuracy of radiation diagnostic modalities in brain tumor: A nonrandomized, nonexperimental, cross-sectional trial,” Medicine, vol. 97, no. 31, p. e11256, 2018, https://doi.org/10.1097/MD.0000000000011256.
- B. Hakyemez, N. Yıldırım, G. Gokalp, C. Erdogan, and M. Parlak, “The contribution of diffusion-weighted MR imaging to distinguishing typical from atypical meningiomas,” Neuroradiology, vol. 48, no. 8, pp. 513–520, 2006, https://doi.org/10.1007/s00234-006-0094-z.
- S. Kathiravan and J. Kanakaraj, “A review of magnetic resonance imaging techniques,” SmartCR, vol. 3, no. 5, pp. 358–366, 2013, https://doi.org/10.6029/smartcr.2013.05.006.
- M. Aamir et al., “A deep learning approach for brain tumor classification using MRI images,” Computers and Electrical Engineering, vol. 101, p. 108105, 2022, https://doi.org/10.1016/j.compeleceng.2022.108105.
- S. Pereira, A. Pinto, V. Alves, and C. A. Silva, “Brain tumor segmentation using convolutional neural networks in MRI images,” IEEE Trans Med Imaging, vol. 35, no. 5, pp. 1240–1251, 2016, https://doi.org/10.1109/TMI.2016.2538465.
- M. Lee, J. H. Kim, W. Choi, and K. H. Lee, “AI-assisted Segmentation Tool for Brain Tumor MR Image Analysis,” Journal of Imaging Informatics in Medicine, vol. 38, no. 1, pp. 74–83, 2025, https://doi.org/10.1007/s10278-024-01187-7.
- S. Sangui, T. Iqbal, P. C. Chandra, S. K. Ghosh, and A. Ghosh, “3D MRI Segmentation using U-Net Architecture for the detection of Brain Tumor,” Procedia Comput Sci, vol. 218, pp. 542–553, 2023, https://doi.org/10.1016/j.procs.2023.01.036.
- F. Isensee et al., “nnu-net: Self-adapting framework for u-net-based medical image segmentation,” arXiv preprint arXiv:1809.10486, 2018, https://doi.org/10.48550/arXiv.1809.10486.
- S. Deepak and P. M. Ameer, “Brain tumor classification using deep CNN features via transfer learning,” Comput Biol Med, vol. 111, p. 103345, 2019, https://doi.org/10.1016/j.compbiomed.2019.103345.
- N. JS, “Brain tumor segmentation using multi-scale attention U-Net with EfficientNetB4 encoder for enhanced MRI analysis,” Sci Rep, vol. 15, no. 1, pp. 1–20, 2025, https://doi.org/10.1038/s41598-025-94267-9.
- T. Yang, X. Lu, L. Yang, M. Yang, J. Chen, and H. Zhao, “Application of MRI image segmentation algorithm for brain tumors based on improved YOLO,” Front Neurosci, vol. 18, p. 1510175, 2025, https://doi.org/10.3389/fnins.2024.1510175.
- P. Kickingereder et al., “Radiomic profiling of glioblastoma: identifying an imaging predictor of patient survival with improved performance over established clinical and radiologic risk models,” Radiology, vol. 280, no. 3, pp. 880–889, 2016, https://doi.org/10.1148/radiol.2016160845.
- M. Cè et al., “Artificial intelligence in brain tumor imaging: a step toward personalized medicine,” Current Oncology, vol. 30, no. 3, pp. 2673–2701, 2023, https://doi.org/10.3390/curroncol30030203.
- M. Karabacak, P. Jagtiani, L. Di, A. H. Shah, R. J. Komotar, and K. Margetis, “Advancing precision prognostication in neuro-oncology: Machine learning models for data-driven personalized survival predictions in IDH-wildtype glioblastoma,” Neurooncol Adv, vol. 6, no. 1, p. vdae096, 2024, https://doi.org/10.1093/noajnl/vdae096.
- B. H. Menze et al., "The Multimodal Brain Tumor Image Segmentation Benchmark (BRATS)," in IEEE Transactions on Medical Imaging, vol. 34, no. 10, pp. 1993-2024, Oct. 2015, https://doi.org/10.1109/TMI.2014.2377694.
- B. Wan, B. Hu, M. Zhao, K. Li, and X. Ye, “Deep learning-based magnetic resonance image segmentation technique for application to glioma,” Front Med (Lausanne), vol. 10, p. 1172767, 2023, https://doi.org/10.3389/fmed.2023.1172767.
- Z. Yi, L. Long, Y. Zeng, and Z. Liu, “Current advances and challenges in radiomics of brain tumors,” Front Oncol, vol. 11, p. 732196, 2021, https://doi.org/10.3389/fonc.2021.732196.
- D. Shen, G. Wu, and H.-I. Suk, “Deep learning in medical image analysis,” Annu Rev Biomed Eng, vol. 19, no. 1, pp. 221–248, 2017, https://doi.org/10.1146/annurev-bioeng-071516-044442.
- Y. Zhan, Y. Hao, X. Wang, and D. Guo, “Advances of artificial intelligence in clinical application and scientific research of neuro-oncology: Current knowledge and future perspectives,” Crit Rev Oncol Hematol, p. 104682, 2025, https://doi.org/10.1016/j.critrevonc.2025.104682.
- A. Esteva et al., “A guide to deep learning in healthcare,” Nat Med, vol. 25, no. 1, pp. 24–29, 2019, https://doi.org/10.1038/s41591-018-0316-z.
- A. Chaddad, C. Desrosiers, and T. Niazi, “Deep radiomic analysis of MRI related to Alzheimer’s disease,” Ieee Access, vol. 6, pp. 58213–58221, 2018, https://doi.org/10.1109/ACCESS.2018.2871977.
- S. K. Zhou et al., “A review of deep learning in medical imaging: Imaging traits, technology trends, case studies with progress highlights, and future promises,” Proceedings of the IEEE, vol. 109, no. 5, pp. 820–838, 2021, https://doi.org/10.1109/JPROC.2021.3054390.
- G. Litjens et al., “A survey on deep learning in medical image analysis,” Med Image Anal, vol. 42, pp. 60–88, 2017, https://doi.org/10.1016/j.media.2017.07.005.
- A. Vaswani et al., “Attention is all you need,” Adv Neural Inf Process Syst, vol. 30, 2017, https://doi.org/10.48550/arXiv.1706.03762.
- A. Hatamizadeh, V. Nath, Y. Tang, D. Yang, H. R. Roth, and D. Xu, “Swin unetr: Swin transformers for semantic segmentation of brain tumors in mri images,” in International MICCAI brainlesion workshop, pp. 272–284, 2021, https://doi.org/10.48550/arXiv.2201.01266.
- F. Isensee, P. F. Jaeger, S. A. A. Kohl, J. Petersen, and K. H. Maier-Hein, “nnU-Net: a self-configuring method for deep learning-based biomedical image segmentation,” Nat Methods, vol. 18, no. 2, pp. 203–211, 2021, https://doi.org/10.1038/s41592-020-01008-z.
- J. Kang, Z. Ullah, and J. Gwak, “MRI-based brain tumor classification using ensemble of deep features and machine learning classifiers,” Sensors, vol. 21, no. 6, p. 2222, 2021, https://doi.org/10.3390/s21062222.
- K. D. Kumari and E. K. Kumar, “A short review on the role of various deep learning techniques for segmenting and classifying brain tumours from MRI images,” International Journal of Advanced Computer Science and Applications, vol. 13, no. 9, 2022, https://doi.org/10.14569/IJACSA.2022.0130995.
- Z. Wang et al., “A hybrid deep learning scheme for MRI-based preliminary multiclassification diagnosis of primary brain tumors,” Front Oncol, vol. 14, p. 1363756, 2024, https://doi.org/10.3389/fonc.2024.1363756.
- S. Iqbal, A. N. Qureshi, J. Li, and T. Mahmood, “On the analyses of medical images using traditional machine learning techniques and convolutional neural networks,” Archives of Computational Methods in Engineering, vol. 30, no. 5, pp. 3173–3233, 2023, https://doi.org/10.1007/s11831-023-09899-9.
- M. Singh, V. Shrimali, and M. Kumar, “Detection and classification of brain tumor using hybrid feature extraction technique,” Multimed Tools Appl, vol. 82, no. 14, pp. 21483–21507, 2023, https://doi.org/10.1007/s11042-022-14088-0.
- M. K. H. Khan et al., “Machine learning and deep learning for brain tumor MRI image segmentation,” Exp Biol Med, vol. 248, no. 21, pp. 1974–1992, 2023, https://doi.org/10.1177/15353702231214259.
- O. Ronneberger, P. Fischer, and T. Brox, “U-net: Convolutional networks for biomedical image segmentation,” in International Conference on Medical image computing and computer-assisted intervention, pp. 234–241, 2015, https://doi.org/10.48550/arXiv.1505.04597.
- R. Missaoui, W. Hechkel, W. Saadaoui, A. Helali, and M. Leo, “Advanced Deep Learning and Machine Learning Techniques for MRI Brain Tumor Analysis: A Review,” Sensors, vol. 25, no. 9, p. 2746, 2025, https://doi.org/10.3390/s25092746.
- H. A. Khan, W. Jue, M. Mushtaq, and M. U. Mushtaq, “Brain tumor classification in MRI image using convolutional neural network,” Mathematical Biosciences and Engineering, 2021, https://doi.org/10.3934/mbe.2020328.
- A. S. Lundervold and A. Lundervold, “An overview of deep learning in medical imaging focusing on MRI,” Zeitschrift fuer medizinische Physik, vol. 29, no. 2, pp. 102–127, 2019, https://doi.org/10.1016/j.zemedi.2018.11.002.
- Y. Zhang and J. Hong, “Challenges of deep learning in cancers,” SAGE Publications Sage CA: Los Angeles, CA, 2023, https://doi.org/10.1177/15330338231173495.
- R. İncir and F. Bozkurt, “Improving brain tumor classification with combined convolutional neural networks and transfer learning,” Knowl Based Syst, vol. 299, p. 111981, 2024, https://doi.org/10.1016/j.knosys.2024.111981.
- G. Al-Rumaihi et al., “Performance Evaluation of Artificial Intelligence Techniques in the Diagnosis of Brain Tumors: A Systematic Review and Meta-Analysis,” Cureus, vol. 17, no. 7, 2025, https://doi.org/10.7759/cureus.88915.
- M. S. I. Khan et al., “Accurate brain tumor detection using deep convolutional neural network,” Comput Struct Biotechnol J, vol. 20, pp. 4733–4745, 2022, https://doi.org/10.1016/j.csbj.2022.08.039.
- S. Dalal et al., “An efficient brain tumor segmentation method based on adaptive moving self-organizing map and fuzzy K-mean clustering,” Sensors, vol. 23, no. 18, p. 7816, 2023, https://doi.org/10.3390/s23187816.
- U. Bhimavarapu, N. Chintalapudi, and G. Battineni, “Brain tumor detection and categorization with segmentation of improved unsupervised clustering approach and machine learning classifier,” Bioengineering, vol. 11, no. 3, p. 266, 2024, https://doi.org/10.3390/bioengineering11030266.
- A. Stadlbauer and A. Meyer-Bäse, “Artificial intelligence in oncology: a topical collection in 2022,” Cancers, vol. 15, no. 4, p. 1065, 2023, https://doi.org/10.3390/cancers15041065.
- S. N. Deepa and B. Arunadevi, “Extreme learning machine for classification of brain tumor in 3D MR images,” Informatologia, vol. 46, no. 2, pp. 111–121, 2013, https://hrcak.srce.hr/106430.
- H. E. M. Abdalla and M. Y. Esmail, "Brain Tumor Detection by using Artificial Neural Network," 2018 International Conference on Computer, Control, Electrical, and Electronics Engineering (ICCCEEE), pp. 1-6, 2018. https://doi.org/10.1109/ICCCEEE.2018.8515763.
- A. N. et al., “K-Means clustering and neural network for object detecting and identifying abnormality of brain tumor,” Soft comput, vol. 23, 2019, https://doi.org/10.1007/s00500-018-3618-7.
- J. Amin, M. Sharif, M. Yasmin, and S. L. Fernandes, “A distinctive approach in brain tumor detection and classification using MRI,” Pattern Recognit Lett, vol. 139, pp. 118–127, 2020, https://doi.org/10.1016/j.patrec.2017.10.036.
- A. Selvapandian and K. Manivannan, “Fusion based Glioma brain tumor detection and segmentation using ANFIS classification,” Comput Methods Programs Biomed, vol. 166, pp. 33–38, 2018, https://doi.org/10.1016/j.cmpb.2018.09.006.
- H. T. Zaw, N. Maneerat and K. Y. Win, "Brain tumor detection based on Naïve Bayes Classification," 2019 5th International Conference on Engineering, Applied Sciences and Technology (ICEAST), pp. 1-4, 2019. https://doi.org/10.1109/ICEAST.2019.8802562.
- A. Stadlbauer et al., “Radiophysiomics: Brain Tumors Classification by Machine Learning and Physiological MRI Data,” Cancers, vol. 14, no. 10, p. 2363, 2022. https://doi.org/10.3390/cancers14102363.
- G. Çınarer and B. G. Emiroğlu, “Classificatin of brain tumors by machine learning algorithms,” in 2019 3rd international symposium on multidisciplinary studies and innovative technologies (ISMSIT), pp. 1–4, 2019, https://doi.org/10.1109/ISMSIT.2019.8932878.
- R. H. Ramdlon, E. M. Kusumaningtyas, and T. Karlita, “Brain tumor classification using MRI images with K-nearest neighbor method,” in 2019 International Electronics Symposium (IES), pp. 660–667, 2019, https://doi.org/10.1109/ELECSYM.2019.8901560.
- B. F. Marghalani and M. Arif, “Automatic classification of brain tumor and Alzheimer’s disease in MRI,” Procedia Comput Sci, vol. 163, pp. 78–84, 2019, https://doi.org/10.1016/j.procs.2019.12.089.
- C. S. Rao and K. Karunakara, “Efficient detection and classification of brain tumor using kernel based SVM for MRI,” Multimed Tools Appl, vol. 81, no. 5, pp. 7393–7417, 2022, https://doi.org/10.1007/s11042-021-11821-z.
- A. K. Mandle, S. P. Sahu, and G. Gupta, “Brain tumor segmentation and classification in MRI using clustering and kernel-based SVM,” Biomedical and Pharmacology Journal, vol. 15, no. 2, pp. 699–716, 2022, https://doi.org/10.13005/bpj/2409.
- P. Kaur, G. Singh, and P. Kaur, “Classification and Validation of MRI Brain Tumor Using Optimised Machine Learning Approach,” In ICDSMLA 2019: Proceedings of the 1st International Conference on Data Science, Machine Learning and Applications, pp. 172–189, 2020, https://doi.org/10.1007/978-981-15-1420-3_19.
- S. Jiang, Y. Gu, and E. Kumar, “Magnetic Resonance Imaging (MRI) Brain Tumor Image Classification Based on Five Machine Learning Algorithms,” Cloud Computing and Data Science, pp. 122–133, 2023, https://doi.org/10.37256/ccds.4220232740.
- A. S. Musallam, A. S. Sherif, and M. K. Hussein, “A new convolutional neural network architecture for automatic detection of brain tumors in magnetic resonance imaging images,” IEEE access, vol. 10, pp. 2775–2782, 2022, https://doi.org/10.1109/ACCESS.2022.3140289.
- D. R. Nayak, N. Padhy, P. K. Mallick, M. Zymbler, and S. Kumar, “Brain tumor classification using dense efficient-net,” Axioms, vol. 11, no. 1, p. 34, 2022, https://doi.org/10.3390/axioms11010034.
- M. R. Obeidavi and K. Maghooli, “Tumor detection in brain MRI using residual convolutional neural networks,” in 2022 International conference on machine vision and image processing (MVIP), pp. 1–5, 2022, https://doi.org/10.1109/MVIP53647.2022.9738767.
- M. A. Mahjoubi, S. Hamida, O. El Gannour, B. Cherradi, A. El Abbassi, and A. Raihani, “Improved multiclass brain tumor detection using convolutional neural networks and magnetic resonance imaging,” Int. J. Adv. Comput. Sci. Appl, vol. 14, no. 3, pp. 406–414, 2023, https://doi.org/10.14569/IJACSA.2023.0140346.
- A. M. Hashan, E. Agbozo, A. A. K. Al-Saeedi, S. Saha, A. Haidari, and M. N. F. Rabi, “Brain tumor detection in MRI images using image processing techniques,” in 2021 4th International Symposium on Agents, Multi-Agent Systems and Robotics (ISAMSR), pp. 24–28, 2021, https://doi.org/10.1109/ISAMSR53229.2021.9567799.
- Y. Bhanothu, A. Kamalakannan, and G. Rajamanickam, “Detection and classification of brain tumor in MRI images using deep convolutional network,” in 2020 6th international conference on advanced computing and communication systems (ICACCS), pp. 248–252, 2020, https://doi.org/10.1109/ICACCS48705.2020.9074375.
- H. M. Rai and K. Chatterjee, “2D MRI image analysis and brain tumor detection using deep learning CNN model LeU-Net,” Multimed Tools Appl, vol. 80, no. 28, pp. 36111–36141, 2021, https://doi.org/10.1007/s11042-021-11504-9.
- C. K. Mohan and S. Samudrala, “MRI Brain Tumor Detection and Classification Using U-NET CNN,” in 2023 International Conference on Data Science and Network Security (ICDSNS), pp. 1–5, 2023, https://doi.org/10.1109/ICDSNS58469.2023.10245128.
- H. R. Almadhoun and S. S. Abu-Naser, “Detection of brain tumor using deep learning,” 2022, https://philpapers.org/rec/ALMDOB.
- M. I. Mahmud, M. Mamun, and A. Abdelgawad, “A Deep Analysis of Brain Tumor Detection from MR Images Using Deep Learning Networks,” Algorithms, vol. 16, no. 4, p. 176. 2023, https://doi.org/10.3390/a16040176.
- S. Kumar and D. Kumar, “Human brain tumor classification and segmentation using CNN,” Multimed Tools Appl, vol. 82, no. 5, pp. 7599–7620, 2023, https://doi.org/10.1007/s11042-022-13713-2.
- S. Asif, M. Zhao, F. Tang, and Y. Zhu, “An enhanced deep learning method for multi-class brain tumor classification using deep transfer learning,” Multimed Tools Appl, vol. 82, no. 20, pp. 31709–31736, 2023, https://doi.org/10.1007/s11042-023-14828-w.
- E. U. Haq, H. Jianjun, K. Li, H. U. Haq, and T. Zhang, “An MRI-based deep learning approach for efficient classification of brain tumors,” J Ambient Intell Humaniz Comput, vol. 14, no. 6, pp. 6697–6718, 2023, https://doi.org/10.1007/s12652-021-03535-9.
- Z. Rasheed et al., “Automated Classification of Brain Tumors from Magnetic Resonance Imaging Using Deep Learning,” Brain sciences, vol. 13, no. 4, p. 602. 2023, https://doi.org/10.3390/brainsci13040602.
- B. K. Joshi, M., & Singh, “Proportion Estimation and Multi-Class Classification of Abnormal Brain Cells,” vol. 1 No. 2, 2024, https://doi.org/10.47852/bonviewMEDIN42021685.
- R. Haque, Md. M. Hassan, A. K. Bairagi, and S. M. Shariful Islam, “NeuroNet19: an explainable deep neural network model for the classification of brain tumors using magnetic resonance imaging data,” Sci Rep, vol. 14, no. 1, p. 1524, 2024, https://doi.org/10.1038/s41598-024-51867-1.
- B. Ch. Mohanty, P. K. Subudhi, R. Dash, and B. Mohanty, “Feature-enhanced deep learning technique with soft attention for MRI-based brain tumor classification,” International Journal of Information Technology, vol. 16, no. 3, pp. 1617–1626, 2024, https://doi.org/10.1007/s41870-023-01701-0.
- S. Jain and V. Jain, “Novel approach to classify brain tumor based on transfer learning and deep learning,” International Journal of Information Technology, vol. 15, no. 4, pp. 2031–2038, 2023, https://doi.org/10.1007/s41870-023-01259-x.
- H. M. Omran, K. Ibrahim, G. T. Abdel-Jaber, and A.-N. Sharkawy, “Brain Tumor Classification from MRI Images Using Hybrid Deep Learning Approaches: VGG19 with SoftMax and SVM Classifiers,” International Journal of Robotics and Control Systems, vol. 6, no. 1, pp. 16–35, 2026, https://doi.org/10.31763/ijrcs.v6i1.2260.
- S. Kumar, S. Choudhary, A. Jain, K. Singh, A. Ahmadian, and M. Y. Bajuri, “Brain tumor classification using deep neural network and transfer learning,” Brain Topogr, vol. 36, no. 3, pp. 305–318, 2023, https://doi.org/10.1007/s10548-023-00953-0.
- A. Saboor et al., “DDFC: deep learning approach for deep feature extraction and classification of brain tumors using magnetic resonance imaging in E-healthcare system,” Sci Rep, vol. 14, no. 1, p. 6425, 2024, https://doi.org/10.1038/s41598-024-56983-6.
- O. Özkaraca et al., “Multiple brain tumor classification with dense CNN architecture using brain MRI images,” Life, vol. 13, no. 2, p. 349, 2023, https://doi.org/https://doi.org/10.3390/life13020349.
AUTHOR BIOGRAPHY
Hanan M. Omran is a Ph.D. researcher in Mechatronics Engineering. She obtained her B.Sc. degree in Mechatronics Engineering from South Valley University in 2013 and earned her M.Sc. degree in the same field in 2018. Her research focuses on the application of artificial intelligence techniques in mechatronic systems, with particular interest in machine learning, deep learning, and robotics. She aims to develop intelligent solutions and data-driven models to enhance performance in industrial and medical applications. She can be contacted at email: enghananomran@gmail.com |
|

| Prof. Dr. Khalil Ibrahim received the B.S. degree in mechanical engineering from Assiut University, Assiut, Egypt, in 2001, and the Ph.D. degree in mechatronics and robotics engineering from Egypt-Japan University of Science and Technology, New Borg El Arab, Egypt, in 2013. He was an Assistant Professor with the Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China. He is currently a full professor with the Department of Mechatronics Engineering at Assiut University's Faculty of Engineering, Egypt. He was the PI of Involving the Industry 4.0 and automation processes to the manufacturing technologies in Egypt. This grant is support by Academic of Scientific Research and Technology (ASRT) and Science and Technology Center of Excellency (STCE), Egypt. Now, my position is the dean of the faculty of industry and energy technological. His research interests include surgical robot systems, a minimally invasive surgery robot, Automatic control, Mechatronics system, Path planning control and Formation control. He can be contacted at email: khalil.ibrahim@aun.edu.eg |
|
|

| Prof. Dr. Gamal T. Abdel-Jaber is President of New Assiut technological university (NATU), Assiut, Egypt, From 1 October 2022. Prof. Gamal was Dean of Faculty of Engineering, South Valley University, Qena, Egypt, from 2011 to September 2022. He is Professor of Machine Design and Tribology Field, Mechanical Engineering Department, Faculty of Engineering, South Valley University. He is the main supervisor of many M.Sc. and PhD. Students in Mechanical Engineering Department in South Valley University, Egypt. He published many research papers in international scientific journals, book chapters and international scientific conferences, in machine design and tribology Field. His research areas of interests are materials and casting technology including bearing material, self-lubricant material, brake and clutch material, and aluminum casting alloys. In addition, techniques including powder metallurgy, casting, and coating. He can be contacted at email: gtag2000@eng.svu.edu.eg |
|
|

| Associate Prof. Dr. Abdel-Nasser Sharkawy     is an associate Professor at Mechatronics Engineering, Mechanical Engineering Department, Faculty of Engineering, Qena University [formerly, South Valley University (SVU)], Qena, Egypt. Sharkawy was graduated with a first-class honors B.Sc. degree in May 2013 and received his M.Sc. degree in April 2016 from Mechatronics Engineering, Mechanical Engineering Department, SVU, Egypt. In March 2020, Sharkawy received his Ph.D. degree from Robotics Group, Department of Mechanical Engineering and Aeronautics, University of Patras, Patras, Greece. Sharkawy has an excellent experience for teaching the under-graduate and postgraduate courses in the field of Mechatronics and Robotics Engineering. Sharkawy has published more than 90 papers in international scientific journals, book chapters and international scientific conferences. His research areas of interest include robotics, human-robot interaction, mechatronic systems, neural networks, machine learning, and control and automation. He can be contacted at email: abdelnassersharkawy@eng.svu.edu.eg is an associate Professor at Mechatronics Engineering, Mechanical Engineering Department, Faculty of Engineering, Qena University [formerly, South Valley University (SVU)], Qena, Egypt. Sharkawy was graduated with a first-class honors B.Sc. degree in May 2013 and received his M.Sc. degree in April 2016 from Mechatronics Engineering, Mechanical Engineering Department, SVU, Egypt. In March 2020, Sharkawy received his Ph.D. degree from Robotics Group, Department of Mechanical Engineering and Aeronautics, University of Patras, Patras, Greece. Sharkawy has an excellent experience for teaching the under-graduate and postgraduate courses in the field of Mechatronics and Robotics Engineering. Sharkawy has published more than 90 papers in international scientific journals, book chapters and international scientific conferences. His research areas of interest include robotics, human-robot interaction, mechatronic systems, neural networks, machine learning, and control and automation. He can be contacted at email: abdelnassersharkawy@eng.svu.edu.eg |
Hanan M. Omran (A Systematic Review of Machine Learning and Deep Learning Approaches in MRI-Based Brain Tumour Analysis, Detection and Classification)