ISSN: 2685-9572 Buletin Ilmiah Sarjana Teknik Elektro
Vol. 7, No. 3, September 2025, pp. 338-349
Transforming EEG into Scalable Neurotechnology: Advances, Frontiers, and Future Directions
Yuri Pamungkas 1, Evi Triandini 2, Adrian Jaleco Forca 3, Thosporn Sangsawang 4, Abdul Karim 5
1 Department of Medical Technology, Institut Teknologi Sepuluh Nopember, Indonesia
2 Department of Information System, Institut Teknologi dan Bisnis STIKOM Bali, Indonesia
3 College of Science and Technology, Guimaras State University, Philippines
4 Division of Educational Technology & Communications, Rajamangala University of Technology Thanyaburi, Thailand
5 Department of Artificial Intelligence Convergence, Hallym University, Republic of Korea
ARTICLE INFORMATION |
| ABSTRACT |
Article History: Received 30 May 2025 Revised 14 July 2025 Accepted 21 July 2025 |
| 
Electroencephalography (EEG) is a key neurotechnology that enables non-invasive, high-temporal resolution monitoring of brain activity. This review examines recent advancements in EEG-based neuroscience from 2021 to 2025, with a focus on applications in neurodegenerative disease diagnosis, cognitive assessment, emotion recognition, and brain-computer interface (BCI) development. Twenty peer-reviewed studies were selected using predefined inclusion criteria, emphasizing the use of machine learning on EEG data. Each study was assessed based on EEG settings, feature extraction, classification models, and outcomes. Emerging trends show increased adoption of advanced computational techniques such as deep learning, capsule networks, and explainable AI for tasks like seizure prediction and psychiatric classification. Applications have expanded to real-world domains including neuromarketing, emotion-aware architecture, and driver alertness systems. However, methodological inconsistencies (ranging from varied preprocessing protocols to inconsistent performance metrics) pose significant challenges to reproducibility and real-world deployment. Technical limitations such as inter-subject variability, low spatial resolution, and artifact contamination were found to negatively impact model accuracy and generalizability. Moreover, most studies lacked transparency regarding bias mitigation, dataset diversity, and ethical safeguards such as data privacy and model interpretability. Future EEG research must integrate multimodal data (e.g., EEG-fNIRS), embrace real-time edge processing, adopt federated learning frameworks, and prioritize personalized, explainable models. Greater emphasis on reproducibility and ethical standards is essential for the clinical translation of EEG-based technologies. This review highlights EEG’s expanding role in neuroscience and emphasizes the need for rigorous, ethically grounded innovation. |
Keywords: Electroencephalography; Neurotechnology; Machine Learning in EEG; Explainable AI; Brain-Computer Interface |
Corresponding Author: Yuri Pamungkas, Institut Teknologi Sepuluh Nopember, Surabaya, Indonesia. Email: yuri@its.ac.id |
This work is open access under a Creative Commons Attribution-Share Alike 4.0 
|
Document Citation: Y. Pamungkas, E. Triandini, A. J. Forca, T. Sangsawang, and A. Karim, “Transforming EEG into Scalable Neurotechnology: Advances, Frontiers, and Future Directions,” Buletin Ilmiah Sarjana Teknik Elektro, vol. 7, no. 3, pp. 338-349, 2025, DOI: 10.12928/biste.v7i3.13824. |
INTRODUCTION
Electroencephalography (EEG) has emerged as a pivotal neurotechnology, offering non-invasive, high-temporal resolution monitoring of brain activity that is foundational to both clinical neurophysiology and cognitive neuroscience research. Since its invention by Hans Berger in the early 20th century, EEG has evolved from analog paper-based recordings to high-density digital systems integrated with sophisticated computational tools [1]. Its ability to capture real-time electrophysiological signals makes EEG uniquely suited for investigating dynamic brain states, such as sensory processing, cognition, sleep cycles, and epileptic events [2][3]. Over the past two decades, significant advances have transformed EEG into a more adaptive and application-oriented modality.
The adoption of machine learning (particularly deep learning, capsule networks, and explainable AI) has enhanced EEG’s potential for predictive modeling in domains such as seizure detection, psychiatric classification, and neurorehabilitation [4]. Additionally, the development of wearable EEG systems, dry electrodes, and wireless telemetry has increased the feasibility of mobile EEG applications in naturalistic environments [5]. Multimodal combinations, such as EEG-fNIRS or EEG-eye-tracking integration, further enrich the interpretability of brain signals by contextualizing electrical activity with hemodynamic or behavioral data [6]. These innovations have been complemented by analytical methods including time-frequency decomposition, source localization, and graph-theoretic network modeling [7].
Despite this progress, the field remains fragmented. Many claims of technological breakthroughs rely on studies with limited sample sizes, non-standardized EEG protocols, or insufficient validation across populations. Persistent issues such as motion artifacts, low spatial resolution, and inter-subject variability continue to hinder reproducibility and clinical translation [8]. Moreover, while AI-driven approaches offer promise, they also introduce concerns regarding model interpretability, fairness, and data privacy (issues often underexplored in existing literature) [9][10].
Although several review papers have addressed the evolution of EEG in isolation or within specific subdomains, few have critically synthesized recent trends from an interdisciplinary, systems-level perspective. This review aims to fill that gap by providing a focused evaluation of EEG-based neuroscience studies published between 2021 and 2025. Specifically, we examine how recent developments in AI integration, multimodal systems, and real-world applications are reshaping the landscape of EEG research. By analyzing the methodological rigor, translational barriers, and ethical implications of contemporary EEG innovation, this review offers a timely roadmap for advancing EEG toward scalable, real-time, and ethically aligned neurotechnologies.
- FUNDAMENTALS OF EEG TECHNOLOGIES
EEG measures fluctuations in electrical potentials generated by ionic currents between neurons, requiring highly sensitive systems due to its microvolt-range signals. Accurate interpretation hinges on minimizing noise and artifacts, making a solid grasp of EEG’s physiological basis essential (particularly for applications in cognitive research and clinical diagnosis of conditions like epilepsy, dementia, and sleep disorders) [11]. Signal quality is influenced by multiple technical factors, including correct electrode placement (e.g., 10–20 system), electrode type (wet or dry), and preprocessing steps such as amplification, filtering, and segmentation. EEG signals span characteristic frequency bands, from delta (deep sleep) to gamma (focused attention), which reflect mental and physiological states. Proper interpretation not only requires knowledge of these bands but also advanced analytical methods like time-frequency analysis, functional connectivity, and machine learning for pattern recognition [12].
Electrode Placement
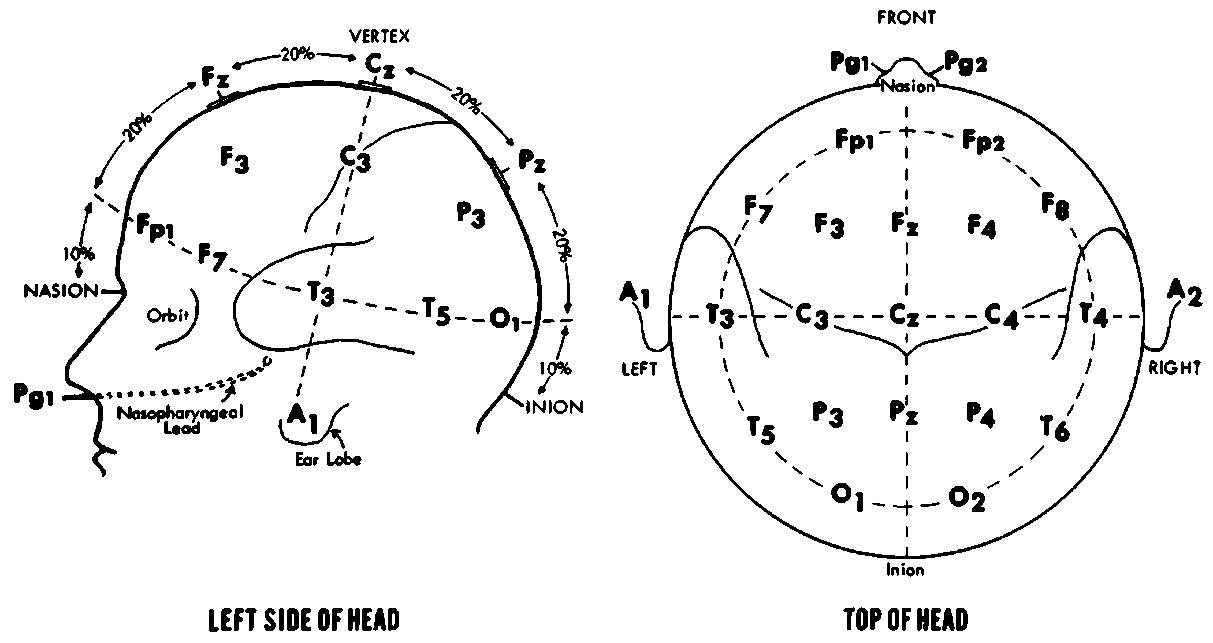
EEG electrode placement is a critical factor in ensuring the quality and accuracy of brain activity recordings. To achieve consistent and reproducible results across individuals and studies, electrodes must be placed systematically on the scalp. The most widely adopted method is the international 10-20 system, which organizes electrodes based on anatomical landmarks such as the nasion, inion, and preauricular points. This system divides the scalp into proportional distances (10% and 20% intervals) ensuring standardization across clinical and research settings. Electrode labels like “F” for the frontal lobe and “z” for the midline enable precise identification of electrode positions (e.g., Fz, F3, F4), supporting consistent localization of brain regions involved in various cognitive and neurological functions [13].
Beyond the standard 10-20 configuration (Figure 1), modern EEG systems increasingly employ high-density electrode arrays, consisting of up to 128 or 256 electrodes. These advanced setups offer enhanced spatial resolution, making them particularly valuable in clinical diagnostics and neuroscience research. High-density arrays allow for more accurate mapping of localized brain activity, which is essential for applications such as identifying epileptic foci, conducting functional brain imaging, and developing brain-computer interfaces (BCIs). The added electrodes capture subtle electrical variations across smaller cortical regions, enabling researchers and clinicians to observe more granular neural dynamics and improve the precision of data interpretation in both experimental and clinical environments [14].

|
(a) Left side of head | (b) Top of head |
Figure 1. EEG electrode 10-20 system [15]
Electrode types and materials also play an important role in the placement and effectiveness of EEG can be seen in Figure 2. The electrodes are typically made of materials like silver/silver chloride (Ag/AgCl) or gold, which have excellent conductivity properties, allowing for efficient detection of the small electrical signals emitted by the brain. More modern EEG systems may also use dry electrodes, which eliminate the need for conductive gel or paste, making them more convenient for patients, especially in long-term monitoring or ambulatory settings. These dry electrodes are often designed for improved comfort and easier placement, as they require less preparation time [16].

| 
| 
| 
|
(a) AgCl wet EEG electrode | (b) Gold wet EEG electrode | (c) AgCl dry EEG electrode | (d) Gold dry EEG electrode |
Figure 2. EEG electrode types and materials [17][18]
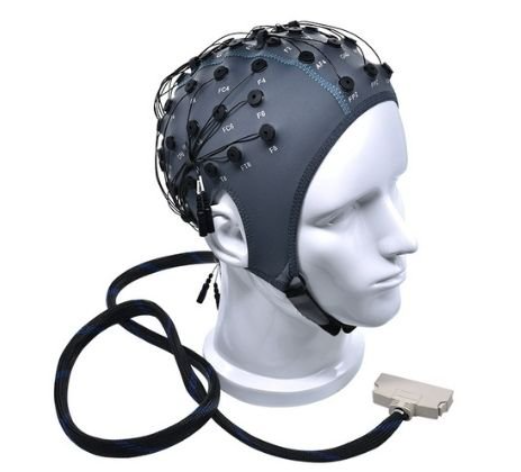
Building upon standardized placement and material innovations, EEG headsets have emerged as practical, user-friendly tools for recording brain activity in both research and applied settings can be seen in Figure 3. These headsets integrate electrodes (either wet or dry) into wearable, often wireless designs that simplify the setup process and improve usability, especially in non-clinical or mobile environments. EEG headsets typically position sensors according to the 10–20 system or its simplified variants, allowing consistent monitoring of cognitive and emotional states. Advances in miniaturized electronics, Bluetooth or Wi-Fi transmission, and onboard signal processing have enabled real-time EEG monitoring with relatively high fidelity [19].
| 
| 
| 
|
(a) "BESDATA" EEG headset | (b) “Emotiv” EEG headset | (c) “Muse” EEG headset | (d) “OpenBCI” EEG headset |
Figure 3. EEG headsets [17],[20]-[22]
Commercially available headsets such as Emotiv, Muse, and OpenBCI exemplify this trend, offering platforms for applications ranging from attention tracking and neurofeedback training to mental workload estimation and brain-computer interface (BCI) development. Although EEG headsets still face limitations in spatial resolution and signal noise compared to high-density systems, their portability, comfort, and accessibility continue to expand the reach of EEG technology into daily life, education, mental health, and consumer neurotechnology domains [23].
Brainwave Frequency Bands
EEG (electroencephalography) signals are categorised based on frequency bands that reflect various forms of electrical oscillation activity in the brain. This division is not only technical in nature, but also highly significant physiologically, as each frequency band is closely related to states of consciousness, cognitive activity, and specific emotional or behavioural states. The five main frequency bands commonly analysed in EEG include delta, theta, alpha, beta, and gamma waves can be seen in Table 1. This classification of EEG frequency bands provides an important framework for understanding the dynamics of human brain activity. Not only used in neuroscience research, EEG spectral analysis also serves as an important diagnostic tool in detecting epilepsy (where abnormal wave patterns may appear in specific bands), sleep disorders, consciousness disorders, and evaluating brain function during anaesthesia or coma. The ability to isolate these frequency bands enables a more precise approach in neuromonitoring and EEG-based neurofeedback interventions.
Table 1. EEG frequency bands [24]
Brainwave | Frequency | Physiological State | Clinical Characteristics & Significance |
Delta | 0.5 – 4 Hz | Deep sleep (non-REM stages 3 & 4), unconsciousness | - High amplitude and lowest frequency.
|
- Dominates during slow-wave sleep.
|
- Presence of delta while awake may indicate diffuse or localized brain dysfunction
|
Theta | 4 – 8 Hz | Light sleep, drowsiness, meditation, wakefulness in children | - Linked to internalized mental activity
|
- Associated with memory encoding and retrieval in adults.
|
- Common in children during wakefulness.
|
Alpha | 8 – 13 Hz | Relaxed, eyes closed but awake | - Most prominent over occipital and parietal lobes.
|
- Decreases with attention or cognitive demand.
|
- Increases during mental relaxation or "wakeful rest".
|
Beta | 13 – 30 Hz | Active thinking, problem-solving, alertness | - Found predominantly in frontal and central regions.
|
- Increased beta may reflect stress or anxiety but also cognitive and motor readiness.
|
- Dominates during focused mental activity.
|
Gamma | 30 – 100 Hz | High-level cognitive processing, attention, consciousness | |
- Important in sensory integration, working memory, and conscious perception.
|
- Detection is challenging due to low amplitude and susceptibility to artifacts.
|
Amplification and Signal Processing in EEG Systems
Electroencephalographic (EEG) signals are inherently minute, typically ranging between 10 and 100 microvolts, which makes them vulnerable to distortion and interference from both physiological and environmental sources. The raw voltage fluctuations recorded by scalp electrodes are therefore too weak to be analyzed directly. To render these signals usable, the first essential step is amplification. Modern EEG systems utilize high-gain, low-noise differential amplifiers that are specifically designed to enhance the signal’s strength while maintaining signal integrity. These amplifiers not only magnify the neural signals but also optimize parameters such as input impedance and common-mode rejection ratio (CMRR), allowing them to effectively suppress external noise (particularly power line interference and other common-mode signals) without introducing additional artifacts or signal degradation [25].
Despite amplification, EEG signals remain susceptible to contamination from various artifacts. Physiological artifacts such as eye blinks, eye movements (electrooculogram, EOG), facial muscle activity (EMG), and cardiac signals (ECG) are common, particularly in awake subjects. Environmental noise, such as electromagnetic interference from nearby electronic devices or fluctuations due to poor electrode contact, can further obscure the desired neural activity. To address these challenges, signal preprocessing techniques are applied. Most systems integrate band-pass filters to retain frequencies of interest (e.g., 0.5–100 Hz) while attenuating irrelevant low- or high-frequency noise. Additionally, notch filters are employed to eliminate 50/60 Hz line noise. For more sophisticated noise sources, EEG systems often rely on computational approaches such as Independent Component Analysis (ICA), Principal Component Analysis (PCA), or regression-based artifact removal algorithms to identify and subtract contaminating components [26].
Following amplification and filtering, the cleaned EEG signal must be converted into a digital format for storage, visualization, and computational analysis. This process is conducted using Analog-to-Digital Converters (ADCs), which sample the analog waveform at regular intervals (commonly 250 Hz to 1000 Hz or higher) and convert it into a discrete numerical representation. High-resolution ADCs, typically operating at 16- to 24-bit resolution, are essential to preserve the fidelity of the signal during digitization. Once in digital form, the EEG data can be processed using various software platforms for real-time monitoring, feature extraction (e.g., power spectral density, event-related potentials), machine learning classification, and long-term archival [27] can be seen in Figure 4.
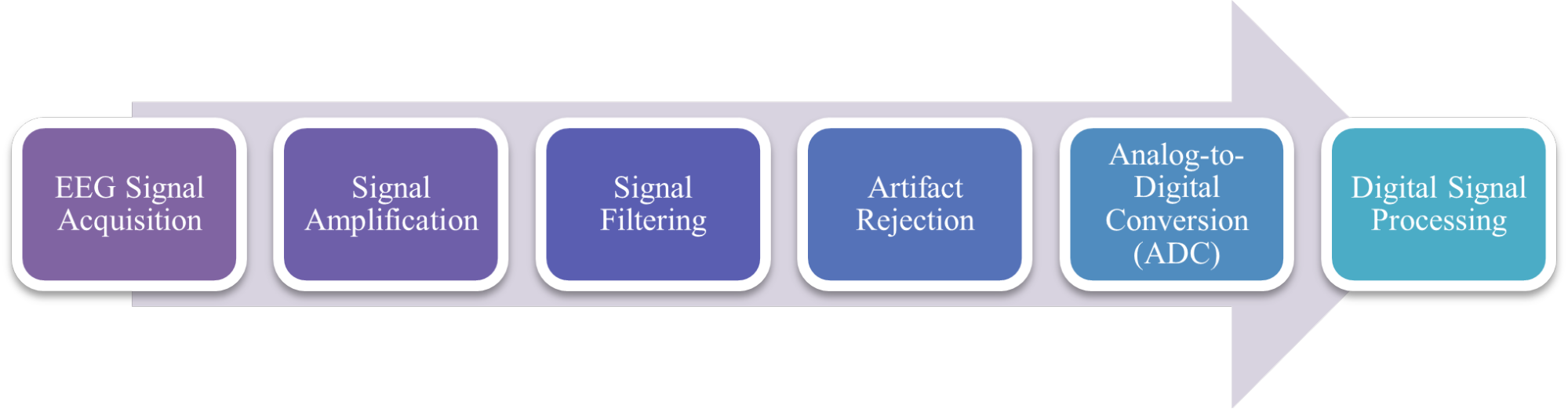
Figure 4. Signal processing in EEG system
Pattern Extraction and Interpretation
Pattern extraction and interpretation is a critical phase that follows the digitization of raw EEG signals, enabling the transformation of complex biosignals into meaningful representations of brain activity. This process is fundamental in both clinical neurophysiology and cognitive neuroscience, as it facilitates the identification of underlying neural mechanisms and the detection of potential pathological alterations. One of the primary approaches in this stage is spectral analysis, which decomposes EEG signals into constituent frequency bands (delta, theta, alpha, beta, and gamma) each corresponding to specific brain states such as sleep, relaxation, attention, or high-level cognitive activity [28]. Methods like Fast Fourier Transform (FFT) and Welch’s method are commonly used to quantify the power distribution within these bands, allowing researchers to assess cognitive states such as alertness, mental workload, or drowsiness [29].
To gain a more dynamic understanding, time-frequency analysis techniques such as Short-Time Fourier Transform (STFT), Wavelet Transform (WT), and Hilbert-Huang Transform (HHT) are employed. These methods enable tracking of how spectral content evolves over time, which is particularly valuable for detecting transient events like epileptic discharges or changes related to cognitive tasks [30]. Beyond frequency-based analyses, a broad array of EEG features is extracted, including statistical metrics (e.g., mean, variance, entropy), connectivity measures (e.g., coherence, phase-locking value), nonlinear characteristics (e.g., fractal dimension, Lyapunov exponent), and hemispheric asymmetry indices.
These features serve as input for machine learning algorithms such as SVM, Random Forests, and deep learning models like CNNs. Such models have shown high accuracy in classifying mental states, detecting neurological disorders such as epilepsy, and identifying demographic traits like age or sex from EEG patterns [31]. Moreover, machine learning extends the interpretative power of EEG by detecting subtle, high-dimensional patterns that are often imperceptible to manual analysis. With the integration of explainable AI (XAI) approaches, researchers can trace model predictions back to specific EEG features or brain regions, enhancing transparency and clinical applicability [32][33].
- ADVANCEMENTS AND APPLICATION OF EEG
In recent years, the application of electroencephalography (EEG) has advanced rapidly across various fields, including neuroengineering, clinical diagnostics, and brain-computer interface (BCI) systems. Between 2021 and 2025 (Table 2), research in EEG has increasingly employed sophisticated computational approaches such as deep learning, self-supervised models, and hybrid frameworks to address complex tasks like epileptic seizure prediction, emotion recognition, and motor activity classification. To provide a comprehensive yet focused overview of these developments, a targeted literature review was conducted, covering studies published between January 2021 and May 2025. The search was performed across five major academic databases: IEEE Xplore, ScienceDirect, and SpringerLink. Boolean keyword combinations used in the search strategy included terms such as (“electroencephalography” OR “EEG”) AND (“deep learning” OR “machine learning” OR “AI”) AND (“neurological disorder” OR “cognitive assessment” OR “mental workload” OR “human-computer interaction”).
Inclusion criteria were defined to select only original, peer-reviewed research articles that utilized human EEG data and applied machine learning or advanced signal processing methods with clearly reported performance outcomes. Studies were excluded if they were reviews, editorials, animal-based research, or lacked methodological transparency. A total of 20 articles met the eligibility criteria and were included in the analysis. For each study, key information was extracted, including the names of the authors, research objectives, EEG acquisition methods (e.g., clinical vs. wearable systems), types of features used, classification approaches, and main findings. Although this review was conducted using a narrative approach, the selection and appraisal process followed a structured logic aligned with best practices for scoping reviews. This methodology aims to ensure transparency, reduce bias, and enhance the relevance of the synthesized insights presented in this paper.
Table 2. EEG research in neuroscience
Ref | Authors & Year | Objective | EEG Type | EEG Data Features | Main Findings |
[34] | Miladinović et al., 2021 | To investigate the correlation between EEG power bands and Parkinson's disease (PD) motor deficit scales | Resting-state Quantitative EEG (qEEG) | Delta (1–4 Hz), Theta (4–8 Hz), Alpha (8–13 Hz), Beta (13–30 Hz); 21 electrodes; 256 Hz sampling; PSD via Welch’s method | Delta positively correlated with FOGQ; Alpha negatively correlated with FOGQ; Theta positively and Beta negatively correlated with UPDRS-III; No correlation with H&Y, BERG, MPAS, 6MWT, TUG |
[35] | Safi et al., 2021 | To improve early Alzheimer’s detection using EEG signals with Hjorth parameters | 20-channel EEG, 10–20 system, 200 Hz | Variance, skewness, kurtosis, Shannon entropy, sure entropy, Hjorth parameters; used DWT, EMD, and band filtering | Combining Hjorth features with DWT and KNN gave the best accuracy at 97.64 percent |
[36] | Molina et al., 2022 | To explore EEG band coupling using complex network analysis in dyslexia diagnosis | Resting-state EEG with auditory stimuli (white noise modulated at 4.8, 16, 40 Hz) | 32-channel EEG, 500 Hz sampling rate, intra-electrode Phase-Amplitude Coupling (PAC) using Modulation Index (MI), bands: Delta to Gamma | Dyslexic children showed altered PAC network topology; notably reduced small-worldness during 4.8 Hz stimulus; small-worldness metric enabled classification with 73% accuracy |
[37] | Gunawardena et al., 2023 | FC analysis & channel selection using manifold learning for AD detection | Resting-state, bipolar montage | 23 bipolar channels, Isomap-GPLVM, kernel similarity matrix | Better than standard FC methods; key changes in occipital–parietal & fronto-parietal connectivity for AD |
[38] | Jung et al., 2023 | To assess emotional and brain responses to biophilic hospital designs | Emotiv Epoc X (14-channel EEG) | Power of theta, alpha, beta, gamma bands in multiple brain regions | Biophilic design increases relaxation and reduces arousal; >90% ML classification accuracy |
[39] | Wąsikowska, 2023 | To study advertising effectiveness using EEG and biometrics | 19-channel EEG (Contec KT88-2400) | Frontal asymmetry, GSR, ECG, ICA-cleaned EEG signals, Z-score averaging | Emotionally engaging ads are better remembered; EEG helps identify scenes with highest engagement. |
[40] | Khalid et al., 2024 | To analyze EEG sub-bands for improved Parkinson’s Disease detection using GRU and PSD features | Resting-state EEG (UCSD dataset) | 32-channel EEG, 512 Hz sampling, PSD from delta, theta, alpha, mu, beta, gamma bands using Welch method | Gamma and beta bands gave highest classification accuracy (up to 98.6%); GRU outperformed SVM and MCNN; alpha, beta, and gamma showed significant discriminatory power |
[41] | Ahmed et al., 2024 | Classify psychiatric disorders using EEG and deep learning models | Resting-state EEG (eyes closed) | 19 channels, 945 subjects, PSD and FC features across 6 frequency bands, 1140 total features | CNN-LSTM, LSTM, Bi-LSTM, KNN, and ANN achieved high accuracies (up to 98.94 percent) in classifying specific disorders like acute stress and adjustment disorder |
[42] | Shafiei et al., 2024 | Evaluate mental workload using coherence and wPLI features | 124-channel EEG | Coherence, wPLI across Brodmann areas | Combining coherence and wPLI improves prediction accuracy of mental workload domains. |
[43] | Khayretdinova et al., 2024 | Predict brain sex using EEG and interpretable ML models | Resting-state EEG (EO & EC) | 4298 qEEG features (PSD, complexity, connectivity) | DCNN achieved 84.1% balanced accuracy and 89% ROC-AUC; left fronto-central & parietal connectivity key. |
[44] | Grootjans et al., 2024 | To highlight how EEG can study social interaction in developmental neuroscience | Lab-based, hyperscanning, mobile EEG | ERP, ERO, theta power, alpha/beta power, inter-brain synchrony | EEG captures social interaction dynamics; ERN modulated by context; inter-brain synchrony reflects relationship closeness and engagement. |
[45] | Yousaf et al., 2024 | To enhance driver attention and road safety using EEG-informed DRL models | EMOTIV EPOC+ (14-channel) | 250 Hz sampling, 3 cognitive states, ICA preprocessing, standardized features | PPO model achieved 99.3% accuracy and outperformed DQN in classifying attention |
[46] | Cataldo et al., 2024 | To evaluate EEG complexity using Multiscale Fuzzy Entropy (MFE) to distinguish AD from healthy controls. | Clinical EEG (resting-state, eyes closed) | 19 channels (10–20 system); 60s signals; band-pass filtered 0.5–30 Hz; analyzed in delta, theta, alpha, beta bands | AD shows lower MFE at short scales, higher at long scales. AD > HC in delta/theta; AD < HC in alpha/beta bands. |
[47] | Sheoran et al., 2025 | Assess sex impact on emotion recognition | 62-channel EEG | PSD (HHT), attention map | Sex affects emotion EEG patterns: females (left), males (right); improves model accuracy |
[48] | Wong et al., 2025 | To develop an interpretable deep learning model for seizure detection using channel-level EEG annotations. | Scalp EEG (TUSZ, RMH datasets) | 1-s single-channel segments; 22 bipolar channels; 250 Hz sampling; bandpass (0.1–60 Hz) and notch filter. | Proposed CNN-Transformer-MLP model achieved AUC 0.93 (TUSZ) and 0.82 (RMH); XAI via DeepSHAP identified key channels with 0.59 localization sensitivity. |
[49] | Catania et al., 2025 | To study brain activity changes during PNES events and rest using EEG microstates | Scalp EEG (21 channels) | 1000 Hz, filtered 2–40 Hz, 4 microstates (A–D), analyzed duration, coverage, GFP | Microstate C (linked to DMN) increases during rest, decreases during PNES events. |
[50] | Dahiya et al., 2025 | To develop an attention-based capsule network (At-CapNet) using EEG-tNIRS data for accurate clinical emotion recognition | 62-channel EEG (NeuroScan) | Differential entropy across five bands, statistical metrics, PSD; mapped to spatiotemporal matrices | At-CapNet using EEG-tNIRS improved recognition by 1.52–14.35%, outperforming existing models in accuracy and computational efficiency |
[51] | Morales et al., 2025 | Review EEG time-frequency in cognitive control development | Event-related EEG | Theta/delta power, ITPS, ICPS | Midfrontal theta reflects control development; time-frequency reveals age effects missed by ERP. |
[52] | Byeon et al., 2025 | To detect epileptic EEG using a brain stimulation model with optimized features | 19-channel EEG | PSI-based topological features, GA-PSO for feature selection, sub-band decomposition | GA-PSO improved classification accuracy up to 91.3%; cross-band features outperform single-band. |
[53] | Colafiglio et al., 2025 | To classify 12 motivational states using EEG and machine learning, under perception and imagery conditions | High-density EEG (128-channels); evaluated with 14 and 18 channel subset | 512 Hz sampling; 12 motivational states; perception vs imagery; ERP-based features; LOSO validation | Perception yielded higher accuracy than imagery; 18 channels slightly better; best accuracy 88% |
Electroencephalography (EEG) continues to serve as a cornerstone in neuroscience research, offering valuable insights into brain activity across a spectrum of neurological, psychiatric, cognitive, and affective domains. Recent studies have increasingly leveraged EEG to investigate neurodegenerative diseases. For instance, Miladinović et al. [34] and Khalid et al. [40] demonstrated that specific EEG frequency bands (particularly delta, theta, beta, and gamma) hold strong diagnostic potential for Parkinson’s disease, while Cataldo et al. [46] employed multiscale fuzzy entropy analysis to differentiate Alzheimer’s patients from healthy controls. Safi et al. [35] further enhanced early Alzheimer’s detection by integrating Hjorth parameters and wavelet-based features, achieving impressive classification accuracy.
Parallel to clinical diagnostics, EEG is gaining ground in cognitive neuroscience and learning disorders. Molina et al. [36] utilized phase-amplitude coupling networks to reveal altered topologies in children with dyslexia. Additionally, Gunawardena et al. [37] and Shafiei et al. [42] explored EEG-based functional connectivity and coherence metrics to improve the detection of Alzheimer’s disease and mental workload states, respectively. The integration of explainable AI (XAI) has also become increasingly prevalent, as seen in Wong et al. [48] who employed a CNN-Transformer-MLP model for seizure detection and used DeepSHAP to enhance interpretability.
In affective neuroscience and applied settings, studies such as Jung et al. [38] and Wąsikowska [39] have used EEG to assess emotional responses to hospital architecture and advertising stimuli, respectively. Meanwhile, Ahmed et al. [41], Khayretdinova et al. [43], and Dahiya et al. [50] have applied deep learning frameworks (including LSTM, DCNN, and capsule networks) to classify psychiatric conditions, predict brain sex, and enhance emotion recognition, often with accuracy rates exceeding 90%. EEG's utility has also extended into social and developmental neuroscience. Grootjans et al. [44] employed hyperscanning to investigate inter-brain synchrony during social interaction, while Catania et al. [49] analyzed microstate dynamics in psychogenic non-epileptic seizures (PNES).
Finally, innovative applications such as those by Yousaf et al. [45] and Byeon et al. [52] illustrate the incorporation of EEG in driver safety systems and epileptic detection using hybrid feature selection models. Studies like Colafiglio et al. [53] show that motivational states can be classified using high-density EEG, even under conditions of mental imagery. Collectively, these findings underscore a vibrant evolution in EEG research, marked by advances in signal acquisition, interpretability, and cross-disciplinary integration (paving the way for more robust, trustworthy, and clinically impactful neurotechnological tools).
- CHALLENGES AND FUTURE DIRECTIONS
- Challenges
Although EEG research in neuroscience has progressed rapidly, its full integration into clinical and real-world applications remains hindered by persistent challenges. These obstacles encompass technical complexities, methodological inconsistencies, and regulatory gaps, all of which must be systematically resolved to enhance the reliability, generalizability, and ethical use of EEG-based systems. One major technical hurdle is the substantial variability observed both between and within individuals, driven by factors such as inconsistent electrode positioning, anatomical differences, fluctuating physiological conditions, and variations in recording equipment. While recent machine learning approaches have achieved high accuracy in specific contexts, their lack of standardization and cross-site validation limits broader applicability. Standardized acquisition protocols and harmonized preprocessing pipelines are urgently needed to improve reproducibility and interoperability across studies.
Signal artifacts remain a persistent problem, especially in real-world or mobile EEG settings. Despite using advanced cleaning techniques such as ICA and filtering, EEG is still highly susceptible to contamination from non-neural sources like muscle movements and eye blinks. These challenges are magnified in wearable systems or naturalistic studies, where movement is difficult to control. To overcome this, future systems must integrate real-time adaptive artifact rejection and potentially incorporate multi-sensor fusion (such as EEG-EMG) to enhance signal quality. At the same time, EEG's strength in temporal resolution is offset by its limited spatial resolution, particularly in accessing subcortical brain activity. While source localization and multimodal integration (e.g., EEG-fMRI, EEG-tNIRS) offer improvements, their cost and complexity restrict clinical use, demanding development of more accessible and affordable multimodal EEG solutions.
Beyond technical hurdles, interpretability of AI models remains a significant barrier to clinical adoption. Deep learning systems for seizure detection and psychiatric diagnosis have shown impressive performance but are often viewed as “black boxes” by clinicians. Although explainable AI (XAI) methods such as DeepSHAP have been proposed to address this, current tools lack validation in clinical workflows. Effective translation will require clinician-in-the-loop systems and domain-specific interpretability strategies to make AI outputs transparent and clinically meaningful. Furthermore, the field is constrained by limited and demographically narrow datasets, which hampers model generalizability. Existing studies often rely on small, homogeneous samples, underlining the urgent need for large, diverse, and well-annotated EEG repositories that can support equitable and robust model training.
Lastly, the ethical, legal, and regulatory dimensions of EEG-AI systems are increasingly pressing. As applications expand into domains such as driver attention monitoring and consumer neurotechnology, challenges around data privacy, informed consent, algorithmic bias, and ownership become more complex. The lack of mature regulatory standards for AI-integrated EEG systems exacerbates these risks. To ensure the safe and responsible deployment of EEG technologies, a multidisciplinary approach involving neuroscientists, clinicians, ethicists, technologists, and policymakers is essential. Such collaboration is critical to building comprehensive, forward-thinking frameworks that balance innovation with safety, transparency, and social accountability.
- Future Directions
To overcome the complex challenges inherent in EEG-based neuroscience, upcoming research efforts should focus on designing hybrid multimodal systems that combine EEG with synergistic technologies like functional near-infrared spectroscopy (fNIRS), eye-tracking, or motion sensing devices. These combinations enhance contextual interpretation and signal reliability, especially in real-world or mobile settings. Furthermore, the adoption of edge computing and low-latency signal processing enables real-time EEG analysis on embedded platforms, supporting responsive applications like seizure monitoring, brain-computer interfaces (BCIs), and adaptive neurofeedback without reliance on cloud-based infrastructure.
Federated learning presents another promising avenue to overcome data sharing and privacy limitations. By allowing decentralized model training across multiple institutions without exposing raw data, federated learning facilitates the development of robust and generalizable EEG models. In parallel, future EEG systems must embrace personalization, where models are tailored to individual users' neurophysiological profiles. Techniques such as transfer learning and adaptive calibration can significantly improve prediction accuracy, particularly in dynamic environments where cognitive states fluctuate rapidly.
Lastly, the field must reinforce its commitment to explainability, reproducibility, and ethical standards. Explainable AI (XAI) frameworks should be refined to generate outputs interpretable by clinicians and aligned with real-world medical decisions. Co-designing EEG technologies with healthcare professionals, validating systems across diverse populations, and publishing open-source data and code are essential steps to foster trust and accelerate clinical translation. Through these strategies, EEG research can evolve into a scalable and ethically sound foundation for next-generation neurotechnological applications.
- CONCLUSIONS
Electroencephalography (EEG) has undergone remarkable development in recent years, solidifying its role as a versatile tool in neuroscience research and clinical applications. The incorporation of cutting-edge signal processing methods (including deep learning, explainable artificial intelligence, and multimodal integration) has greatly expanded EEG’s range of applications, from diagnosing neurological conditions to monitoring emotional and cognitive states, as well as facilitating interaction between humans and computational systems. The studies reviewed between 2021 and 2025 demonstrate how EEG continues to evolve from a diagnostic instrument into a dynamic platform for real-time, individualized brain assessment and intervention. However, this progress is accompanied by several enduring challenges. Technical issues such as signal variability, artifact contamination, and limited spatial resolution persist, particularly in mobile and real-world settings. Methodologically, the reliance on non-standardized protocols and limited datasets undermines model generalizability. Moreover, the complexity and opacity of AI-driven EEG systems raise critical concerns regarding clinical interpretability and ethical deployment. The lack of demographic diversity in datasets and underdeveloped regulatory frameworks further complicate the translation of EEG innovations into scalable and trustworthy applications.
To navigate these challenges, future EEG research must adopt integrated strategies. This includes the development of hybrid systems that combine EEG with modalities like fNIRS or eye-tracking, the use of edge computing for low-latency processing, and the implementation of federated learning to preserve data privacy. Personalization of EEG models and co-design with clinicians will also be essential to enhance accuracy, acceptance, and clinical relevance. Additionally, rigorous efforts must be made to improve explainability, reproducibility, and ethical transparency. In sum, EEG stands at the intersection of neuroscience, engineering, and artificial intelligence. With sustained interdisciplinary collaboration and commitment to addressing its current limitations, EEG is poised to become a cornerstone of next-generation neurotechnology (offering scalable, real-time, and ethically aligned solutions for brain health, cognitive research, and human augmentation).
DECLARATION
Author Contribution
All authors contributed equally to the main contributor to this paper. All authors read and approved the final paper.
Acknowledgement
The authors would like to acknowledge the Department of Medical Technology, Institut Teknologi Sepuluh Nopember, for the facilities and support in this research. The authors also gratefully acknowledge financial support from the Institut Teknologi Sepuluh Nopember for this work, under project scheme of the Publication Writing and IPR Incentive Program (PPHKI) 2025.
Conflicts of Interest
The authors declare no conflict of interest.
REFERENCES
- A. Arjoonsingh, B. C. Jamal, and L. Ganti, “History and Evolution of the Electroencephalogram,” Cureus, vol. 16, no. 8, 2024, https://doi.org/10.7759/cureus.66385.
- H. Zhang et al., “The applied principles of EEG analysis methods in neuroscience and clinical neurology,” Military Medical Research, vol. 10, no. 1, p. 67, 2023, https://doi.org/10.1186/s40779-023-00502-7.
- C. M. Michel and M. M. Murray, “Towards the utilization of EEG as a brain imaging tool,” NeuroImage, vol. 61, no. 2, pp. 371–385, 2012, https://doi.org/10.1016/j.neuroimage.2011.12.039.
- J. R. McLaren, D. Yuan, S. Beniczky, M. B. Westover, and F. A. Nascimento, “The future of EEG education in the era of artificial intelligence,” Epilepsia, 2025, doi: https://doi.org/10.1111/epi.18326.
- M. Mohamed, N. Mohamed, and J. G. Kim, “Advancements in Wearable EEG Technology for Improved Home-Based Sleep Monitoring and Assessment: A Review,” Biosensors, vol. 13, no. 12, pp. 1019–1019, 2023, https://doi.org/10.3390/bios13121019.
- L. Qiu, Y. Zhong, Q. Xie, Z. He, X. Wang, Y. Chen, C. A. Zhan, and J. Pan, “Multi-Modal Integration of EEG-fNIRS for Characterization of Brain Activity Evoked by Preferred Music,” Frontiers in Neurorobotics, vol. 16, 2022, doi: https://doi.org/10.3389/fnbot.2022.823435.
- Z. Liu, J. Shore, M. Wang, F. Yuan, A. Buss, and X. Zhao, “A systematic review on hybrid EEG/fNIRS in brain-computer interface,” Biomedical Signal Processing and Control, vol. 68, p. 102595, 2021, https://doi.org/10.1016/j.bspc.2021.102595.
- S. Yun, “Advances, challenges, and prospects of electroencephalography-based biomarkers for psychiatric disorders: a narrative review,” Journal of Yeungnam Medical Science, vol. 41, no. 4, pp. 261–268, 2024, https://doi.org/10.12701/jyms.2024.00668.
- I. Rakhmatulin, M.-S. Dao, A. Nassibi, and D. Mandic, “Exploring Convolutional Neural Network Architectures for EEG Feature Extraction,” Sensors, vol. 24, no. 3, pp. 877–877, 2024, https://doi.org/10.3390/s24030877.
- C. Halkiopoulos, E. Gkintoni, A. Aroutzidis, and H. Antonopoulou, “Advances in Neuroimaging and Deep Learning for Emotion Detection: A Systematic Review of Cognitive Neuroscience and Algorithmic Innovations,” Diagnostics, vol. 15, no. 4, p. 456, 2025, https://doi.org/10.3390/diagnostics15040456.
- A. S. Fahandari, S. Moshiryan, and A. Goshvarpour, “Diagnosis of Cognitive and Mental Disorders: A New Approach Based on Spectral–Spatiotemporal Analysis and Local Graph Structures of Electroencephalogram Signals,” Brain Sciences, vol. 15, no. 1, pp. 68–68, 2025, https://doi.org/10.3390/brainsci15010068.
- E. T. Attar, “Review of electroencephalography signals approaches for mental stress assessment,” Neurosciences, vol. 27, no. 4, pp. 209–215, 2022, https://doi.org/10.17712/nsj.2022.4.20220025.
- E. Gkintoni, A. Aroutzidis, H. Antonopoulou, and C. Halkiopoulos, “From Neural Networks to Emotional Networks: A Systematic Review of EEG-Based Emotion Recognition in Cognitive Neuroscience and Real-World Applications,” Brain Sciences, vol. 15, no. 3, p. 220, 2025, https://doi.org/10.3390/brainsci15030220.
- Y. Sun, X. Chen, B. Liu, L. Liang, Y. Wang, S. Gao, and X. Gao, “Signal acquisition of brain-computer interfaces: A medical-engineering crossover perspective review,” Fundamental research, vol. 5, no. 1, pp. 3-16, 2024, https://doi.org/10.1016/j.fmre.2024.04.011.
- Engineers Community, “Electrode 10-20 system,” Engineers Community, 2018. https://engineerscommunity.com/t/electrode-10-20-system/5486.
- H. Kim, E. Kim, C. Choi, and W.-H. Yeo, “Advances in Soft and Dry Electrodes for Wearable Health Monitoring Devices,” Micromachines, vol. 13, no. 4, p. 629, 2022, https://doi.org/10.3390/mi13040629.
- “EEG Electrodes Manufacturer - FDA, CE, ISO Certified Supplier,” BESDATA, 2024. https://besdatatech.com/eeg-electrodes/.
- “Hot Sale EEG Electrode Reusabe Dry Comb Electrodes AgCl EEG Electrode,” www.alibaba.com, 2021. https://www.alibaba.com/product-detail/Hot-Sale-EEG-Electrode-Reusabe-Dry_1600620280344.html.
- H. Luo, H. Li, W. Tao, Y. Yang, C.-I. Ieong, and F. Wan, “A Portable and Affordable Four-Channel EEG System for Emotion Recognition with Self-Supervised Feature Learning,” Mathematics, vol. 13, no. 10, p. 1608, 2025, https://doi.org/10.3390/math13101608.
- “EEG Headset,” EMOTIV. https://www.emotiv.com/blogs/glossary/eeg-headset
- “MUSE Data Collection,” Krigolson Lab, 2016. https://www.krigolsonlab.com/muse-data-collection.html
- “The Complete Ultracortex,” OpenBCI Online Store, 2015. https://shop.openbci.com/products/the-complete-headset-eeg
- C. Orovas, T. Sapounidis, C. Volioti, and E. Keramopoulos, “EEG in Education: A Scoping Review of Hardware, Software, and Methodological Aspects,” Sensors, vol. 25, no. 1, pp. 182–182, 2024, https://doi.org/10.3390/s25010182.
- Z. Wang and P. Mengoni, “Seizure classification with selected frequency bands and EEG montages: a Natural Language Processing approach,” Brain Informatics, vol. 9, no. 1, 2022, https://doi.org/10.1186/s40708-022-00159-3.
- M. T. Knierim, M. G. Bleichner, and P. Reali, “A Systematic Comparison of High-End and Low-Cost EEG Amplifiers for Concealed, Around-the-Ear EEG Recordings,” Sensors, vol. 23, no. 9, p. 4559, 2023, https://doi.org/10.3390/s23094559.
- A. Chaddad, Y. Wu, Reem Kateb, and A. Bouridane, “Electroencephalography Signal Processing: A Comprehensive Review and Analysis of Methods and Techniques,” Sensors, vol. 23, no. 14, pp. 6434–6434, 2023, https://doi.org/10.3390/s23146434.
- M. Saeidi, W. Karwowski, F. V. Farahani, K. Fiok, R. Taiar, P. A. Hancock, and A. A.-Juaid, “Neural Decoding of EEG Signals with Machine Learning: A Systematic Review,” Brain Sciences, vol. 11, no. 11, p. 1525, 2021, https://doi.org/10.3390/brainsci11111525.
- S. Acharya, A. Khosravi, D. Creighton, R. Alizadehsani, and U. R. Acharya, “Neurostressology: A systematic review of EEG-based automated mental stress perspectives,” Information Fusion, vol. 124, p. 103368, 2025, https://doi.org/10.1016/j.inffus.2025.103368.
- F. B. Ardecani, A. Kumar, S. Sabeti, and O. Shoghli, “Neural correlates of augmented reality safety warnings: EEG analysis of situational awareness and cognitive performance in roadway work zones,” Safety Science, vol. 185, pp. 106802–106802, 2025, https://doi.org/10.1016/j.ssci.2025.106802.
- P.-P. Yuan, J. Zhang, J.-Q. Feng, H.-H. Wang, W.-X. Ren, and C. Wang, “An improved time-frequency analysis method for structural instantaneous frequency identification based on generalized S-transform and synchroextracting transform,” Engineering Structures, vol. 252, p. 113657, 2022, https://doi.org/10.1016/j.engstruct.2021.113657.
- N. Koirala et al., “Assistive Artificial Intelligence in Epilepsy and Its Impact on Epilepsy Care in Low- and Middle-Income Countries,” Brain Sciences, vol. 15, no. 5, p. 481, 2025, https://doi.org/10.3390/brainsci15050481.
- M. T. Khosroshahi et al., “Explainable Artificial Intelligence in Neuroimaging of Alzheimer’s Disease,” Diagnostics, vol. 15, no. 5, pp. 612–612, 2025, https://doi.org/10.3390/diagnostics15050612.
- A. Farizal, A. D. Wibawa, D. P. Wulandari and Y. Pamungkas, "Investigation of Human Brain Waves (EEG) to Recognize Familiar and Unfamiliar Objects Based on Power Spectral Density Features," 2023 International Seminar on Intelligent Technology and Its Applications (ISITIA), pp. 77-82, , 2023, https://doi.org/10.1109/isitia59021.2023.10221052.
- A. Miladinović, M. Ajčević, P. Busan, J. Jarmolowska, M. Deodato, S. Mezzarobba, P. P. Battaglini, and A. Accardo, “EEG changes and motor deficits in Parkinson’s disease patients: Correlation of motor scales and EEG power bands,” Procedia Computer Science, vol. 192, pp. 2616–2623, 2021, https://doi.org/10.1016/j.procs.2021.09.031.
- M. S. Safi and S. M. M. Safi, “Early detection of Alzheimer’s disease from EEG signals using Hjorth parameters,” Biomedical Signal Processing and Control, vol. 65, p. 102338, 2021, https://doi.org/10.1016/j.bspc.2020.102338.
- N. J. G.-Molina, A. Ortiz, F. J. M.-Murcia, M. A. Formoso, and A. Giménez, “Complex network modeling of EEG band coupling in dyslexia: An exploratory analysis of auditory processing and diagnosis,” Knowledge-Based Systems, vol. 240, p. 108098, 2022, https://doi.org/10.1016/j.knosys.2021.108098.
- R. Gunawardena, P. G. Sarrigiannis, D. J. Blackburn, and F. He, “Kernel-based Nonlinear Manifold Learning for EEG-based Functional Connectivity Analysis and Channel Selection with Application to Alzheimer’s Disease,” Neuroscience, vol. 523, pp. 140–156, 2023, https://doi.org/10.1016/j.neuroscience.2023.05.033.
- D. Jung, D. I. Kim, and N. Kim, “Bringing nature into hospital architecture: Machine learning-based EEG analysis of the biophilia effect in virtual reality,” Journal of Environmental Psychology, vol. 89, pp. 102033–102033, 2023, https://doi.org/10.1016/j.jenvp.2023.102033.
- B. Wąsikowska, “The use of electroencephalography (EEG) in a study into the effectiveness of advertising communication,” Procedia computer science, vol. 225, pp. 2477–2486, 2023, https://doi.org/10.1016/j.procs.2023.10.239.
- N. Khalid and M. S. Ehsan, “Critical analysis of Parkinson’s disease detection using EEG sub-bands and gated recurrent unit,” Engineering Science and Technology, an International Journal, vol. 59, p. 101855, 2024, https://doi.org/10.1016/j.jestch.2024.101855.
- Z. Ahmed, A. Wali, S. Shahid, S. Zikria, J. Rasheed, and T. Asuroglu, “Psychiatric disorders from EEG signals through deep learning models,” IBRO Neuroscience Reports, vol. 17, pp. 300–310, 2024, https://doi.org/10.1016/j.ibneur.2024.09.003.
- S. B. Shafiei, S. Shadpour, and A. Shafqat, “Mental Workload evaluation using weighted phase lag index and coherence features extracted from EEG data,” Brain Research Bulletin, vol. 214, pp. 110992–110992, 2024, https://doi.org/10.1016/j.brainresbull.2024.110992.
- M. Khayretdinova, I. Zakharov, P. Pshonkovskaya, T. Adamovich, A. Kiryasov, A. Zhdanov, and A. Shovkun, “Prediction of brain sex from EEG: using large-scale heterogeneous dataset for developing a highly accurate and interpretable ML model,” NeuroImage, vol. 285, pp. 120495–120495, 2023, https://doi.org/10.1016/j.neuroimage.2023.120495.
- Y. Grootjans et al., “Getting closer to social interactions using electroencephalography in developmental cognitive neuroscience,” Developmental Cognitive Neuroscience, vol. 67, pp. 101391–101391, May 2024, https://doi.org/10.1016/j.dcn.2024.101391.
- M. Yousaf, M. Farhan, Y. Saeed, M. J. Iqbal, F. Ullah, and G. Srivastava, “Enhancing driver attention and road safety through EEG-informed deep reinforcement learning and soft computing,” Applied Soft Computing, vol. 167, p. 112320, 2024, https://doi.org/10.1016/j.asoc.2024.112320.
- A. Cataldo, S. Criscuolo, E. D. Benedetto, A. Masciullo, M. Pesola, J. Picone, and R. Schiavoni, “EEG complexity-based algorithm using Multiscale Fuzzy Entropy: Towards a detection of Alzheimer’s disease,” Measurement, vol. 225, pp. 114040–114040, 2023, https://doi.org/10.1016/j.measurement.2023.114040.
- A. Sheoran and C. E. Valderrama, “Impact of sex differences on subject-independent EEG-based emotion recognition models,” Computers in Biology and Medicine, vol. 190, p. 110036, 2025, https://doi.org/10.1016/j.compbiomed.2025.110036.
- S. Wong, A. Simmons, J. R.-Villicana, S. Barnett, S. Sivathamboo, P. Perucca, Z. Ge, P. Kwan, L. Kuhlmann, and T. J. O’Brien, “Channel-annotated deep learning for enhanced interpretability in EEG-based seizure detection,” Biomedical Signal Processing and Control, vol. 103, pp. 107484–107484, 2025, https://doi.org/10.1016/j.bspc.2024.107484.
- C. Catania et al., “EEG microstates during resting-state and dissociative events in patients with psychogenic non-epileptic seizures,” Clinical Neurophysiology, vol. 173, pp. 124–131, 2025, https://doi.org/10.1016/j.clinph.2025.03.002.
- R. Dahiya, G. Mamatha, S. S. Jawale, S. Das, S. Choudhary, V. M. Rathod, and B. J. Rajput, “Deep learning-based multi-brain capsule network for Next-Gen Clinical Emotion recognition using EEG signals,” Neuroscience Informatics, vol. 5, no. 2, p. 100203, 2025, https://doi.org/10.1016/j.neuri.2025.100203.
- S. Morales and G. A. Buzzell, “EEG time-frequency dynamics of early cognitive control development,” Developmental Cognitive Neuroscience, vol. 73, p. 101548, 2025, https://doi.org/10.1016/j.dcn.2025.101548.
- H. Byeon, U. Mahajan, A. Kumar, V. R. Krishna, M. Soni, and M. Bansal, “EEG signal based brain stimulation model to detect epileptic neurological disorders,” Neuroscience Informatics, pp. 100186–100186, 2025, https://doi.org/10.1016/j.neuri.2025.100186.
- T. Colafiglio, A. Lombardi, T. D. Noia, M. Luigia, F. Narducci, and A. M. Proverbio, “Machine learning classification of motivational states: Insights from eeg analysis of perception and imagery,” Expert Systems with Applications, pp. 127076–127076, 2025, https://doi.org/10.1016/j.eswa.2025.127076.
Yuri Pamungkas (Transforming EEG into Scalable Neurotechnology: Advances, Frontiers, and Future Directions)